MINISTRY OF EDUCATION AND SCIENCE OF THE REPUBLIC OF KAZAKHSTAN
KAZAKH NATIONAL WOMEN TEACHER TRAINING UNIVERSITY
INSTITUTE OF NATURAL SCIENCES
DEPARTMENT OF CHEMISTRY

SIW
Topic: Isomerism and Isomerism types
Reviewed by: Sarsenbaeva Zamira Berkbayevna
Performed by: 2nd year student of the specialty
"6B01507-Chemistry" Berdimatova Umida
Almaty 2022
Plan:
-
Introduction
1.1. А.M.Butlerovts chemical, structural theory
II. The main part
2.1. Isomerism of organic compounds
2.2. Types of isomerism.
2.3. Structural isomerism
2.4. Spatial isomerism
III. Conclusion
IV. References
Introduction
Before the emergence of AM Butlerov's theory of chemical structure, the existence of substances with the same composition and molecular weight, but different in the location of atoms, remained unknown. These substances have different properties. The ability of carbon atoms to form four covalent bonds with other carbon atoms opens up the possibility of several compounds with the same elemental composition. This phenomenon is called isomerism.
Prior to the development of the theory of chemical structure, only one substance of C4H10 butane with a linear structure of the carbon chain was known. AM Butlerov suggested that the molecular formula is the same, but the sequence of carbon atoms in the molecule can be a different substance. Thus, an isomer of butane was obtained, which is called isobutane (with a branched structure).
In September 1861, at a conference of naturalists in Speyer (Germany), AM Butlerov spoke on "The chemical structure of matter." In this report, he presented the main ideas of the theory of construction. The essence of this theory consists of the following basic principles:
1. All atoms in a molecule are connected in sequence according to their valences. The order of addition of atoms in a molecule and the nature of the bonds between them M. Butlerov called it chemical structure. It is determined that there are one, two and three bonds between and that the chain of carbon atoms is open and closed:
2. Properties of substances are their qualitative, quantitative,not only in the atoms of the 6ipre molecule the order of communication with each other and between them.
This principle of the theory of construction is an organic, widespread phenomenon of isomerism in chemistry.
3. By studying the properties of a given substance, it is possible to determine the structure of the molecule of the substance and to some extent express the structure and properties of the formula 6ip.
4. Atoms and groups of atoms entering the molecule interact with each other, including the interaction of directly connected atoms.
Butlerov's idea of the interaction of these atoms was further developed by his student VV Markovnikov.
2.1. Isomerism of organic compounds
Organic compounds are chemical compounds (except for carbon oxides, carbon dioxide and its salts) that always contain a carbon atom as the main element. From ancient times the people used natural dyes, cane sugar, various oils, etc. could use. The number of organic compounds has exceeded 5 million due to the chemical interaction of carbon atoms with atoms of many other elements. They are characterized by the phenomenon of isomerism, which studies organic chemistry, and various complex changes.
Before the advent of the theory of the chemical structure of A. M. Butlerov, the existence of substances that have the same composition and the same molecular weight, but differ in the arrangement of atoms, remained unknown. These substances have different properties. The ability of carbon atoms to form four covalent bonds, including those with other carbon atoms, opens up the possibility of the existence of several compounds of the same elemental composition. This phenomenon was called isomerism.
Before the creation of the theory of the chemical structure, only one substance of the composition `C4H10` was known - butane, which has a linear structure of the carbon chain. A. M. Butlerov suggested the possibility of the existence of another substance with the same molecular formula, but with a different sequence of carbon atoms in the molecule. Thus, an isomer of butane was obtained, which was called isobutane (has a branched structure).

There are three isomers for pentane:

The boiling points of butane and pentane differ from each other, which serves as evidence that the properties of the compounds depend on the structure of their molecules.
2.2. Types of isomerism.
Isomerism. Structural and spatial formulas and models According to the theory of chemical structure, each substance has a specific chemical structure. However, several substances can correspond to the same molecular formula. But their chemical structure and structural formula are different. Isomers are substances with the same qualitative and quantitative composition and molecular weight, but different structure and, accordingly, different properties. The order in which carbon atoms interact with each other or with atoms of other elements is called the phenomenon of isomerism. Therefore, ethyl alcohol and dimethyl ether were considered isomers. The phenomenon of isomerism is very common in organic chemistry and is one of the main reasons for the abundance of organic matter.
Isomerism means that each compound has the same molecular formula, but each compound contains two or more substances with different structures. In these substances, called isomers, all the elements are in the same proportions, but each molecule has a different structure of atoms.
The word isomer comes from the Greek word isomers, which means "equal parts". Contrary to the prediction, isomers may or may not have similar characteristics depending on the functional groups in their structure.
There are two main classes of isomerism: constitutional (or structural) isomerism and stereoisomerism (or spatial isomerism). Isomerism is found in both organic substances (alcohols, ketones, etc.) and in inorganic substances (coordination compounds).
Sometimes they appear spontaneously; In these cases, the isomers of the molecule are stable and under normal conditions (25°C,1 atm), which was a very important breakthrough in chemistry when it was discovered.
2.3. Structural isomerism
Structural isomers - compounds of the same qualitative and quantitative composition, differing in the order of binding of atoms, i.e. chemical structure.Therefore, structural isomers have the same molecular formula but different structural formulas.
Types of structural isomerism
-
The isomerism of the carbon skeleton is due to the different order of atoms that form the skeleton of molecules..For example, structural isomers of the composition C5H12:
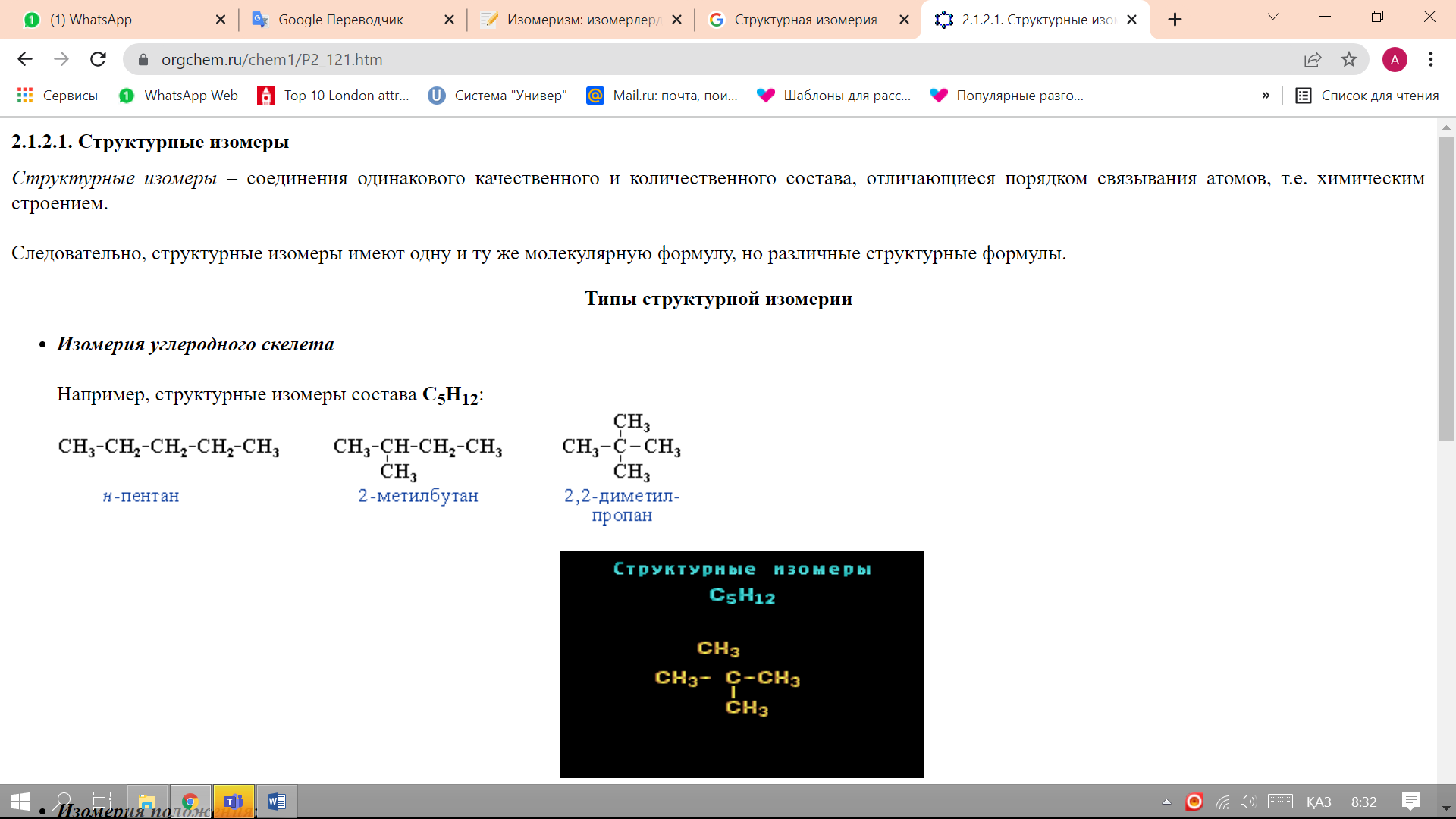
-
Position isomerism:
-
multiple bonds - a type of isomerism characteristic of compounds with more than 3 carbon atoms and having double or triple bonds.
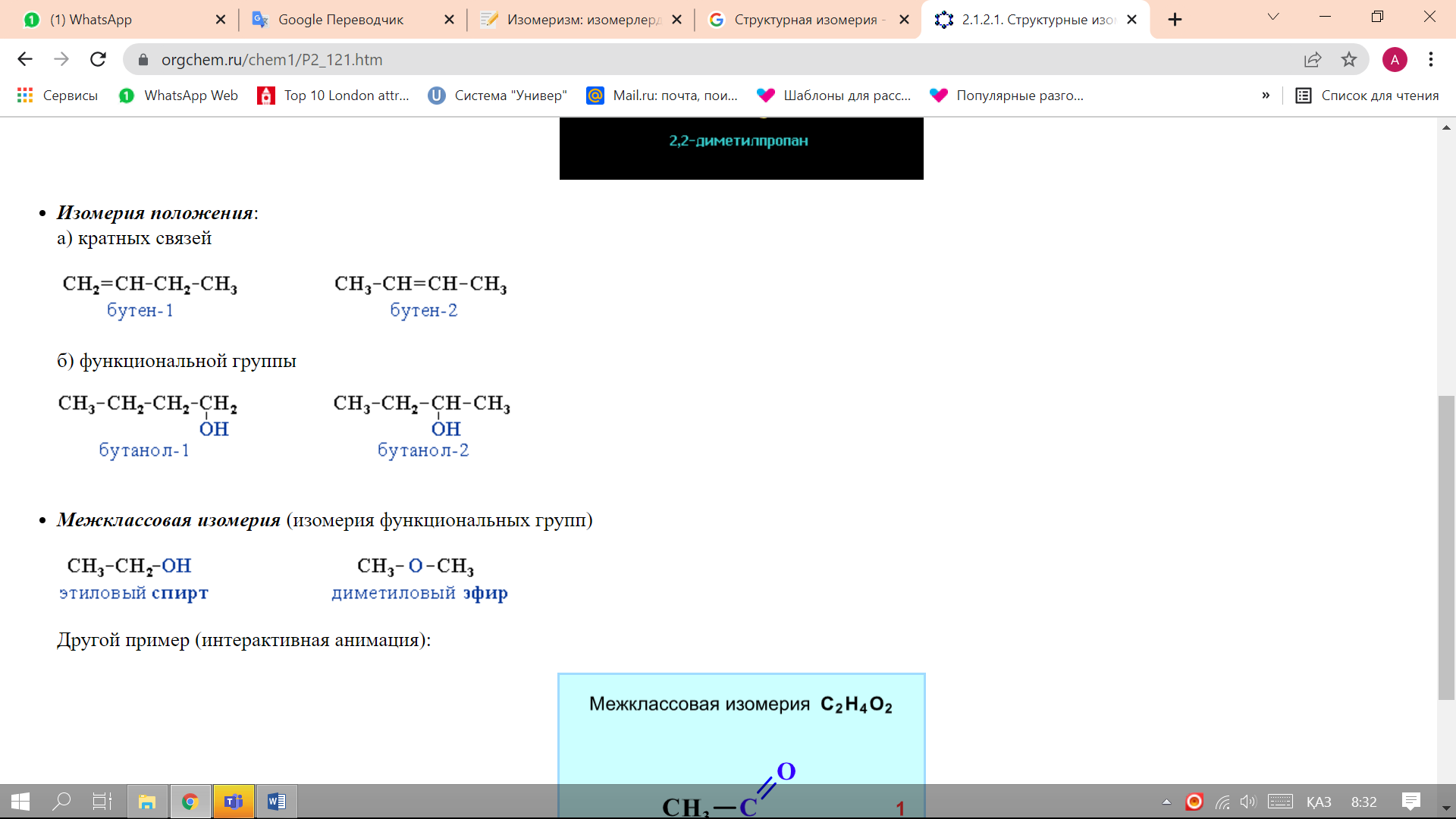
-
functional group- a type of isomerism characteristic of oxygen- and nitrogen-containing organic compounds.

-
Interclass isomerism (isomerism of functional groups) is characteristic of compounds that have the same general formula, but belong to different classes and have different chemical and physical properties.

2.4. Spatial isomerism
Spatial isomers are substances with the same composition and chemical structure, but with a different spatial arrangement of atoms in a molecule. Types of spatial isomerism - geometric (cis-trans) and optical isomerism.
1. Geometric isomerism (or cis-trans-isomerism).
Geometric isomerism is characteristic of compounds in which the position of substituents relative to the plane of the double bond or cycle is different. For example, for alkenes and cycloalkanes.
The double bond does not have free rotation around its axis.
Therefore, substituents at carbon atoms in a double bond can be located either on one side of the double bond plane (cis isomer), or on opposite sides of the double bond plane (trans isomer). In this case, no rotation can be obtained from the cis-isomer of the trans-isomer, and vice versa.
For example, butene-2 exists as cis- and trans-isomers.
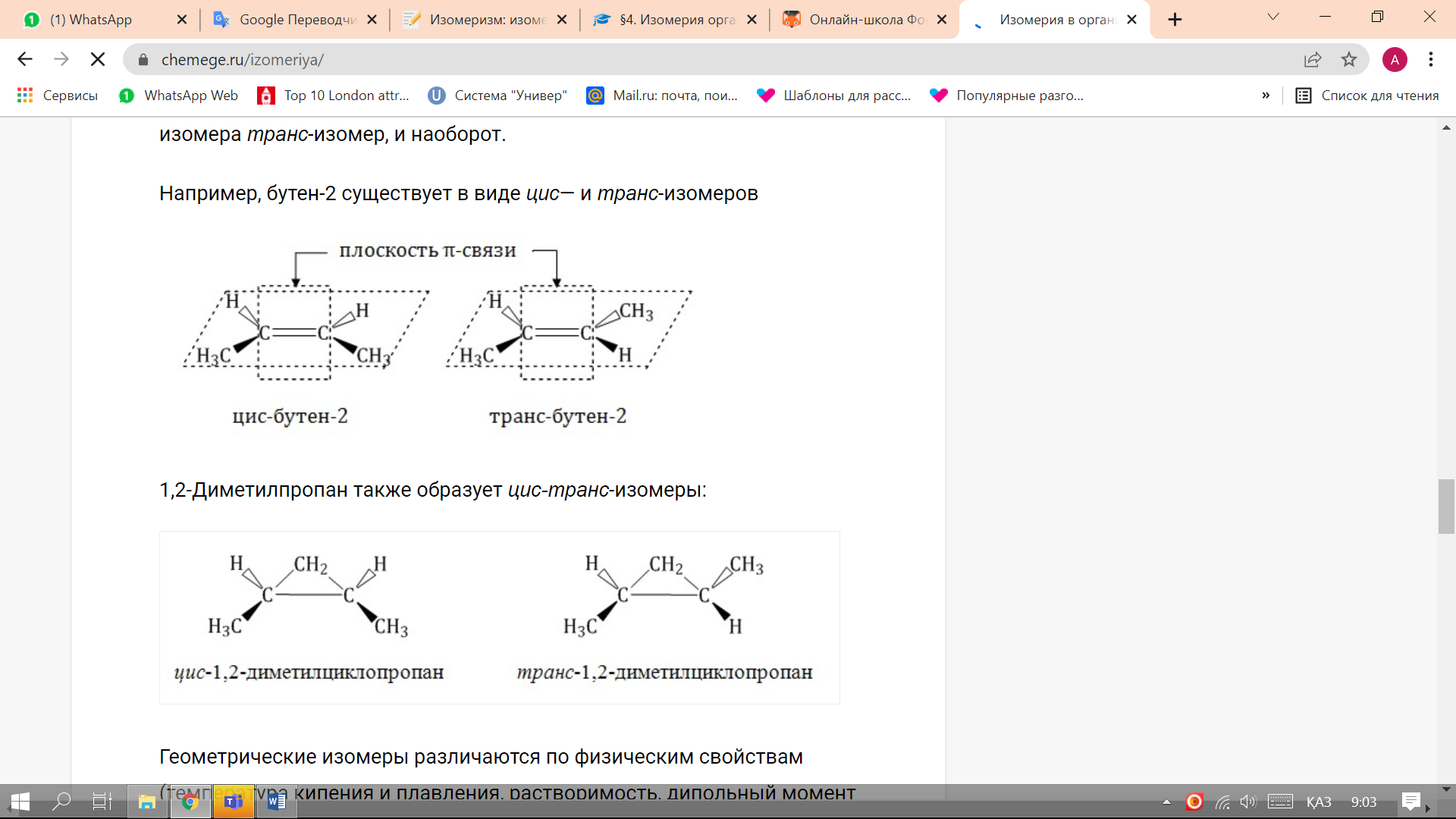
1,2-Dimethylpropane also forms cis-trans isomers:

Geometric isomers differ in physical properties (boiling and melting points, solubility, dipole moment, etc.). For example, the boiling point of cis-butene-2 is 3.73°C, and of trans-butene-2 0.88°C.
At the same time, cis-trans isomerism is characteristic of compounds in which each carbon atom in the C=C double bond (or in the cycle) has two different substituents.
For example, in the butene-1 molecule CH2=CH-CH2-CH3, the substituents at the first carbon atom in the double bond (two hydrogen atoms) are the same, and butene-1 does not form cis-trans isomers. But in the butene-2 CH3-CH=CH-CH3 molecule, the substituents on each carbon atom in the double bond are different (hydrogen atom and methyl group CH3), so butene-2 forms cis- and trans-isomers.
Thus, for compounds of the type СH2=СHR and СR2=СHR', cis-trans isomerism is not typical.
2. Optical isomerism
Optical isomers are spatial isomers, the molecules of which are related to each other as an object and an incompatible mirror image.Optical isomerism is characteristic of molecules of substances having an asymmetric carbon atom. An asymmetric carbon atom is a carbon atom bonded to four different substituents.
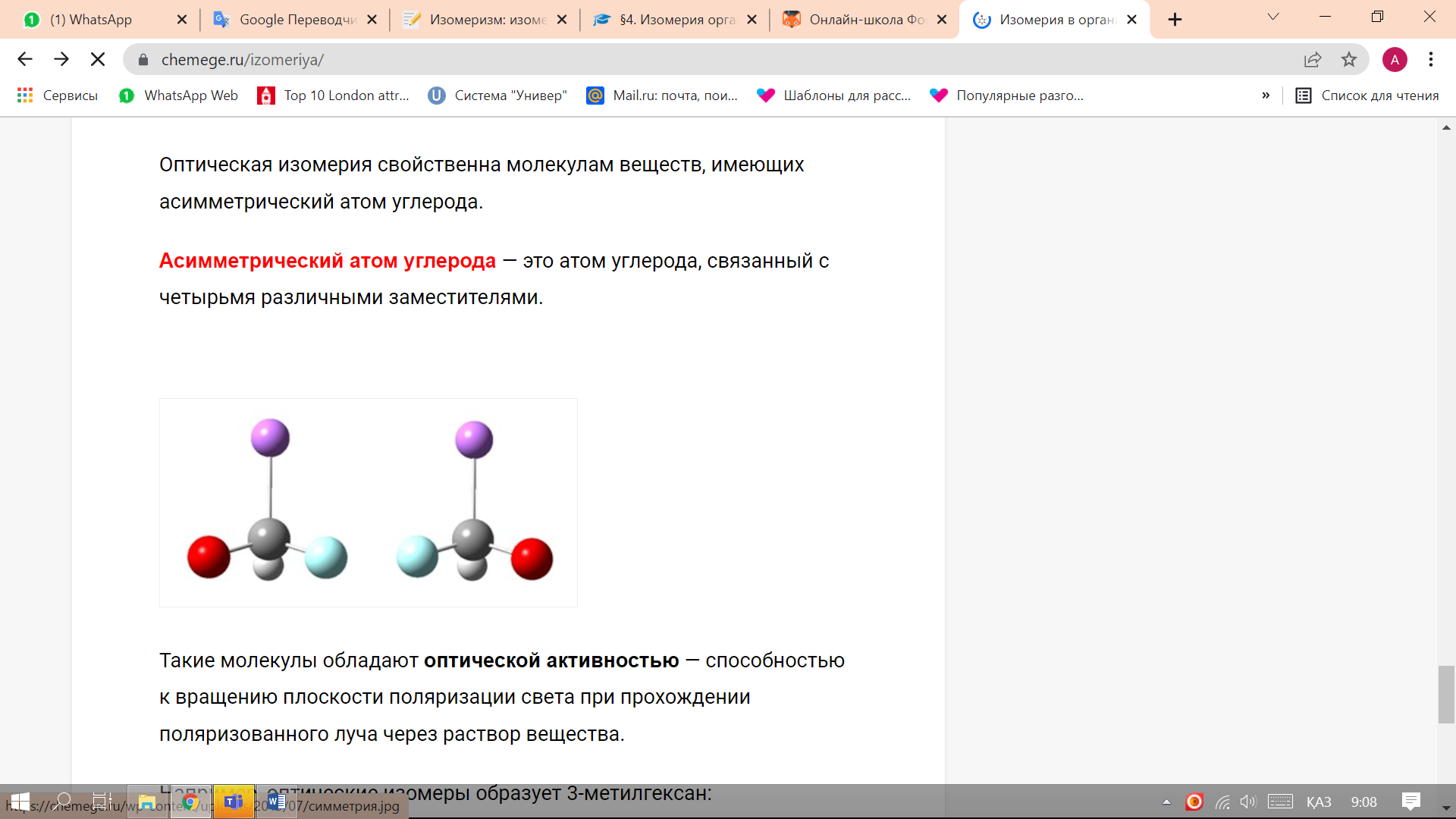
Such molecules have optical activity - the ability to rotate the plane of polarization of light when a polarized beam passes through a solution of a substance. For example, 3-methylhexane forms optical isomers:
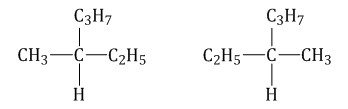
Conclusion
In short, isomers are substances with the same molecular formula, but different structure and different physical and chemical properties.
There are several types of isomerism. Structural isomerism is caused by a different order of addition of carbon atoms in the molecule. Structural isomers may differ in the structure of the carbon skeleton, the plural bonds and the location of substituents, as well as may belong to different classes of organic compounds.
Spatial isomerism arises from the different positions of atoms in space. The order of atomic bonds of geometric isomers is the same, but differs in the spatial arrangement that determines their different properties. Optical isomerism is characteristic of substances containing an asymmetric carbon atom (c), that is, a carbon atom attached to four different substituents. Optical isomers do not match their mirror image.
References
-
Rudzitis G.E. Chemistry. Fundamentals of General Chemistry. Grade 10: textbook for educational institutions: basic level / G. E. Rudzitis, F. G. Feldman. – 14th edition. – M.: Enlightenment, 2012.[ RUSSIAN]
-
Chemistry. Grade 10. Profile level: textbook. for general education institutions / V. V. Eremin, N. E. Kuzmenko, V. V. Lunin et al. - M.: Bustard, 2008. - 463 p. .[ RUSSIAN]
-
Chemistry. Grade 11. Profile level: textbook. for general education institutions / V. V. Eremin, N. E. Kuzmenko, V. V. Lunin et al. - M.: Bustard, 2010. - 462 p. .[ RUSSIAN]
-
Khomchenko G. P., Khomchenko I. G. Collection of problems in chemistry for applicants to universities. - 4th ed. - M.: RIA "New Wave": Publisher Umerenkov, 2012. - 278 p. .[ RUSSIAN]
жүктеу мүмкіндігіне ие боласыз
Бұл материал сайт қолданушысы жариялаған. Материалдың ішінде жазылған барлық ақпаратқа жауапкершілікті жариялаған қолданушы жауап береді. Ұстаз тілегі тек ақпаратты таратуға қолдау көрсетеді. Егер материал сіздің авторлық құқығыңызды бұзған болса немесе басқа да себептермен сайттан өшіру керек деп ойласаңыз осында жазыңыз
Изомерия және түрлері
Изомерия және түрлері
MINISTRY OF EDUCATION AND SCIENCE OF THE REPUBLIC OF KAZAKHSTAN
KAZAKH NATIONAL WOMEN TEACHER TRAINING UNIVERSITY
INSTITUTE OF NATURAL SCIENCES
DEPARTMENT OF CHEMISTRY

SIW
Topic: Isomerism and Isomerism types
Reviewed by: Sarsenbaeva Zamira Berkbayevna
Performed by: 2nd year student of the specialty
"6B01507-Chemistry" Berdimatova Umida
Almaty 2022
Plan:
-
Introduction
1.1. А.M.Butlerovts chemical, structural theory
II. The main part
2.1. Isomerism of organic compounds
2.2. Types of isomerism.
2.3. Structural isomerism
2.4. Spatial isomerism
III. Conclusion
IV. References
Introduction
Before the emergence of AM Butlerov's theory of chemical structure, the existence of substances with the same composition and molecular weight, but different in the location of atoms, remained unknown. These substances have different properties. The ability of carbon atoms to form four covalent bonds with other carbon atoms opens up the possibility of several compounds with the same elemental composition. This phenomenon is called isomerism.
Prior to the development of the theory of chemical structure, only one substance of C4H10 butane with a linear structure of the carbon chain was known. AM Butlerov suggested that the molecular formula is the same, but the sequence of carbon atoms in the molecule can be a different substance. Thus, an isomer of butane was obtained, which is called isobutane (with a branched structure).
In September 1861, at a conference of naturalists in Speyer (Germany), AM Butlerov spoke on "The chemical structure of matter." In this report, he presented the main ideas of the theory of construction. The essence of this theory consists of the following basic principles:
1. All atoms in a molecule are connected in sequence according to their valences. The order of addition of atoms in a molecule and the nature of the bonds between them M. Butlerov called it chemical structure. It is determined that there are one, two and three bonds between and that the chain of carbon atoms is open and closed:
2. Properties of substances are their qualitative, quantitative,not only in the atoms of the 6ipre molecule the order of communication with each other and between them.
This principle of the theory of construction is an organic, widespread phenomenon of isomerism in chemistry.
3. By studying the properties of a given substance, it is possible to determine the structure of the molecule of the substance and to some extent express the structure and properties of the formula 6ip.
4. Atoms and groups of atoms entering the molecule interact with each other, including the interaction of directly connected atoms.
Butlerov's idea of the interaction of these atoms was further developed by his student VV Markovnikov.
2.1. Isomerism of organic compounds
Organic compounds are chemical compounds (except for carbon oxides, carbon dioxide and its salts) that always contain a carbon atom as the main element. From ancient times the people used natural dyes, cane sugar, various oils, etc. could use. The number of organic compounds has exceeded 5 million due to the chemical interaction of carbon atoms with atoms of many other elements. They are characterized by the phenomenon of isomerism, which studies organic chemistry, and various complex changes.
Before the advent of the theory of the chemical structure of A. M. Butlerov, the existence of substances that have the same composition and the same molecular weight, but differ in the arrangement of atoms, remained unknown. These substances have different properties. The ability of carbon atoms to form four covalent bonds, including those with other carbon atoms, opens up the possibility of the existence of several compounds of the same elemental composition. This phenomenon was called isomerism.
Before the creation of the theory of the chemical structure, only one substance of the composition `C4H10` was known - butane, which has a linear structure of the carbon chain. A. M. Butlerov suggested the possibility of the existence of another substance with the same molecular formula, but with a different sequence of carbon atoms in the molecule. Thus, an isomer of butane was obtained, which was called isobutane (has a branched structure).

There are three isomers for pentane:

The boiling points of butane and pentane differ from each other, which serves as evidence that the properties of the compounds depend on the structure of their molecules.
2.2. Types of isomerism.
Isomerism. Structural and spatial formulas and models According to the theory of chemical structure, each substance has a specific chemical structure. However, several substances can correspond to the same molecular formula. But their chemical structure and structural formula are different. Isomers are substances with the same qualitative and quantitative composition and molecular weight, but different structure and, accordingly, different properties. The order in which carbon atoms interact with each other or with atoms of other elements is called the phenomenon of isomerism. Therefore, ethyl alcohol and dimethyl ether were considered isomers. The phenomenon of isomerism is very common in organic chemistry and is one of the main reasons for the abundance of organic matter.
Isomerism means that each compound has the same molecular formula, but each compound contains two or more substances with different structures. In these substances, called isomers, all the elements are in the same proportions, but each molecule has a different structure of atoms.
The word isomer comes from the Greek word isomers, which means "equal parts". Contrary to the prediction, isomers may or may not have similar characteristics depending on the functional groups in their structure.
There are two main classes of isomerism: constitutional (or structural) isomerism and stereoisomerism (or spatial isomerism). Isomerism is found in both organic substances (alcohols, ketones, etc.) and in inorganic substances (coordination compounds).
Sometimes they appear spontaneously; In these cases, the isomers of the molecule are stable and under normal conditions (25°C,1 atm), which was a very important breakthrough in chemistry when it was discovered.
2.3. Structural isomerism
Structural isomers - compounds of the same qualitative and quantitative composition, differing in the order of binding of atoms, i.e. chemical structure.Therefore, structural isomers have the same molecular formula but different structural formulas.
Types of structural isomerism
-
The isomerism of the carbon skeleton is due to the different order of atoms that form the skeleton of molecules..For example, structural isomers of the composition C5H12:

-
Position isomerism:
-
multiple bonds - a type of isomerism characteristic of compounds with more than 3 carbon atoms and having double or triple bonds.

-
functional group- a type of isomerism characteristic of oxygen- and nitrogen-containing organic compounds.

-
Interclass isomerism (isomerism of functional groups) is characteristic of compounds that have the same general formula, but belong to different classes and have different chemical and physical properties.

2.4. Spatial isomerism
Spatial isomers are substances with the same composition and chemical structure, but with a different spatial arrangement of atoms in a molecule. Types of spatial isomerism - geometric (cis-trans) and optical isomerism.
1. Geometric isomerism (or cis-trans-isomerism).
Geometric isomerism is characteristic of compounds in which the position of substituents relative to the plane of the double bond or cycle is different. For example, for alkenes and cycloalkanes.
The double bond does not have free rotation around its axis.
Therefore, substituents at carbon atoms in a double bond can be located either on one side of the double bond plane (cis isomer), or on opposite sides of the double bond plane (trans isomer). In this case, no rotation can be obtained from the cis-isomer of the trans-isomer, and vice versa.
For example, butene-2 exists as cis- and trans-isomers.

1,2-Dimethylpropane also forms cis-trans isomers:

Geometric isomers differ in physical properties (boiling and melting points, solubility, dipole moment, etc.). For example, the boiling point of cis-butene-2 is 3.73°C, and of trans-butene-2 0.88°C.
At the same time, cis-trans isomerism is characteristic of compounds in which each carbon atom in the C=C double bond (or in the cycle) has two different substituents.
For example, in the butene-1 molecule CH2=CH-CH2-CH3, the substituents at the first carbon atom in the double bond (two hydrogen atoms) are the same, and butene-1 does not form cis-trans isomers. But in the butene-2 CH3-CH=CH-CH3 molecule, the substituents on each carbon atom in the double bond are different (hydrogen atom and methyl group CH3), so butene-2 forms cis- and trans-isomers.
Thus, for compounds of the type СH2=СHR and СR2=СHR', cis-trans isomerism is not typical.
2. Optical isomerism
Optical isomers are spatial isomers, the molecules of which are related to each other as an object and an incompatible mirror image.Optical isomerism is characteristic of molecules of substances having an asymmetric carbon atom. An asymmetric carbon atom is a carbon atom bonded to four different substituents.

Such molecules have optical activity - the ability to rotate the plane of polarization of light when a polarized beam passes through a solution of a substance. For example, 3-methylhexane forms optical isomers:
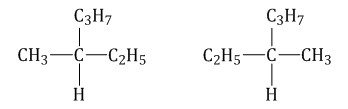
Conclusion
In short, isomers are substances with the same molecular formula, but different structure and different physical and chemical properties.
There are several types of isomerism. Structural isomerism is caused by a different order of addition of carbon atoms in the molecule. Structural isomers may differ in the structure of the carbon skeleton, the plural bonds and the location of substituents, as well as may belong to different classes of organic compounds.
Spatial isomerism arises from the different positions of atoms in space. The order of atomic bonds of geometric isomers is the same, but differs in the spatial arrangement that determines their different properties. Optical isomerism is characteristic of substances containing an asymmetric carbon atom (c), that is, a carbon atom attached to four different substituents. Optical isomers do not match their mirror image.
References
-
Rudzitis G.E. Chemistry. Fundamentals of General Chemistry. Grade 10: textbook for educational institutions: basic level / G. E. Rudzitis, F. G. Feldman. – 14th edition. – M.: Enlightenment, 2012.[ RUSSIAN]
-
Chemistry. Grade 10. Profile level: textbook. for general education institutions / V. V. Eremin, N. E. Kuzmenko, V. V. Lunin et al. - M.: Bustard, 2008. - 463 p. .[ RUSSIAN]
-
Chemistry. Grade 11. Profile level: textbook. for general education institutions / V. V. Eremin, N. E. Kuzmenko, V. V. Lunin et al. - M.: Bustard, 2010. - 462 p. .[ RUSSIAN]
-
Khomchenko G. P., Khomchenko I. G. Collection of problems in chemistry for applicants to universities. - 4th ed. - M.: RIA "New Wave": Publisher Umerenkov, 2012. - 278 p. .[ RUSSIAN]

шағым қалдыра аласыз