Ашық сабақ "Атом құрылысы" презентациясы
Ашық сабақ "Атом құрылысы" презентациясы


#1 слайд
8.1 Electron arrangement .
A том құрылысы
1 слайд
8.1 Electron arrangement . A том құрылысы

#2 слайд
Lesson objectives:
Сабақ мақсаты
•
8.1.3.1 бірінші 20 элементтің электрондар санын анықтау
•
8.1.3.2 электрондардың қабаттарда орналасуының
схемасын салу
•
8.1.3.3 электрондар атомда ядродан арақашықтықтары
артып келе жатқан орбитальдарда біртіндеп
орналасатындығын түсіну
2 слайд
Lesson objectives: Сабақ мақсаты • 8.1.3.1 бірінші 20 элементтің электрондар санын анықтау • 8.1.3.2 электрондардың қабаттарда орналасуының схемасын салу • 8.1.3.3 электрондар атомда ядродан арақашықтықтары артып келе жатқан орбитальдарда біртіндеп орналасатындығын түсіну
#3 слайд
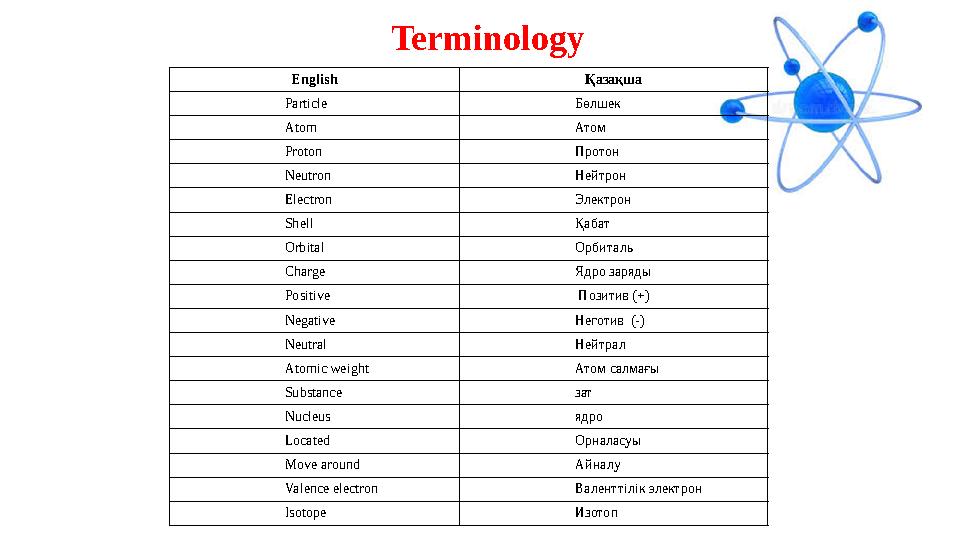
Terminology
English Қазақша
Particle Бөлшек
Atom Атом
Proton Протон
Neutron Нейтрон
Electron Электрон
Shell Қабат
Orbital Орбиталь
Charge Ядро заряды
Positive Позитив (+)
Negative Неготив (-)
Neutral Нейтрал
Atomic weight Атом салмағы
Substance зат
Nucleus ядро
Located Орналасуы
Move around Айналу
Valence electron Валенттілік электрон
Isotope Изотоп
3 слайд
Terminology English Қазақша Particle Бөлшек Atom Атом Proton Протон Neutron Нейтрон Electron Электрон Shell Қабат Orbital Орбиталь Charge Ядро заряды Positive Позитив (+) Negative Неготив (-) Neutral Нейтрал Atomic weight Атом салмағы Substance зат Nucleus ядро Located Орналасуы Move around Айналу Valence electron Валенттілік электрон Isotope Изотоп
#4 слайд
electron
4 слайд
electron
#5 слайд
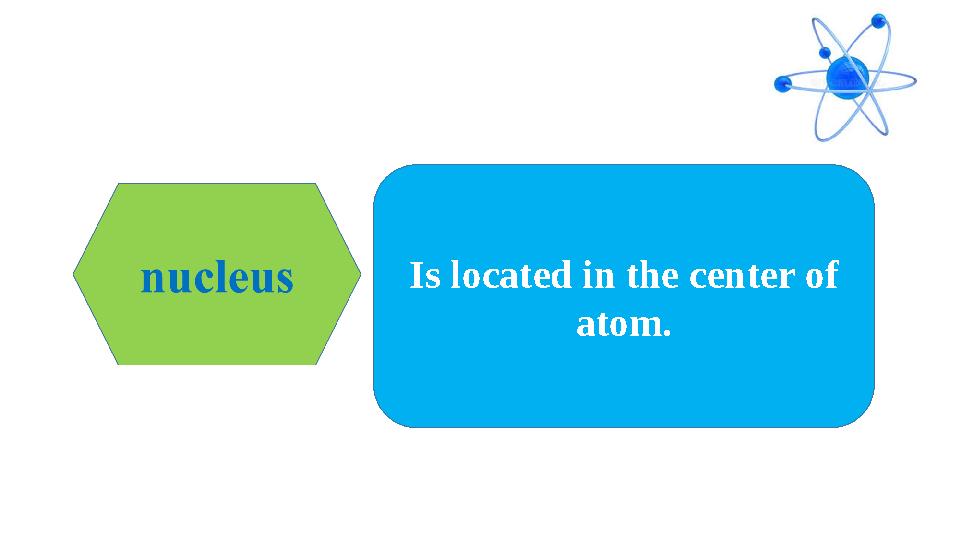
Is located in the center of
atom.
5 слайд
Is located in the center of atom.
#6 слайд
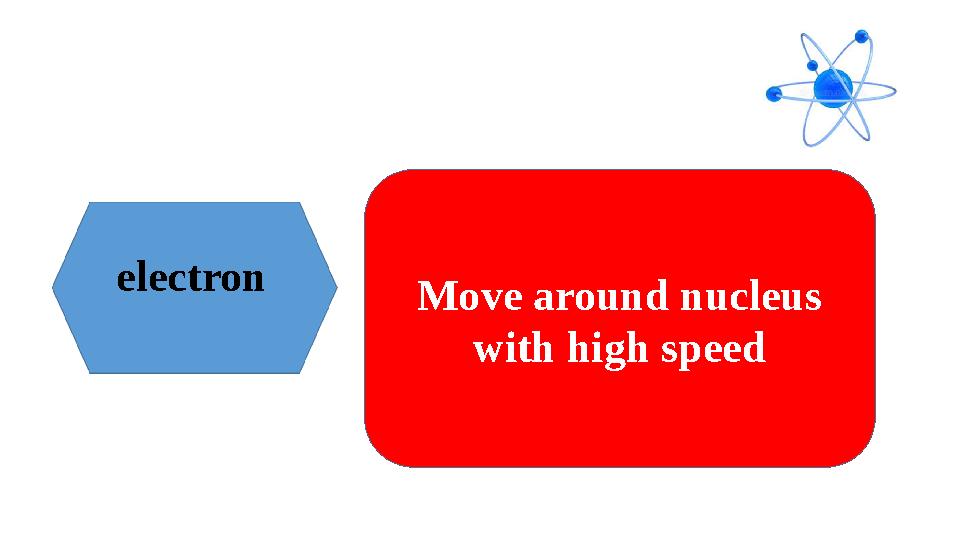
electron
Move around nucleus
with high speed
6 слайд
electron Move around nucleus with high speed
#7 слайд
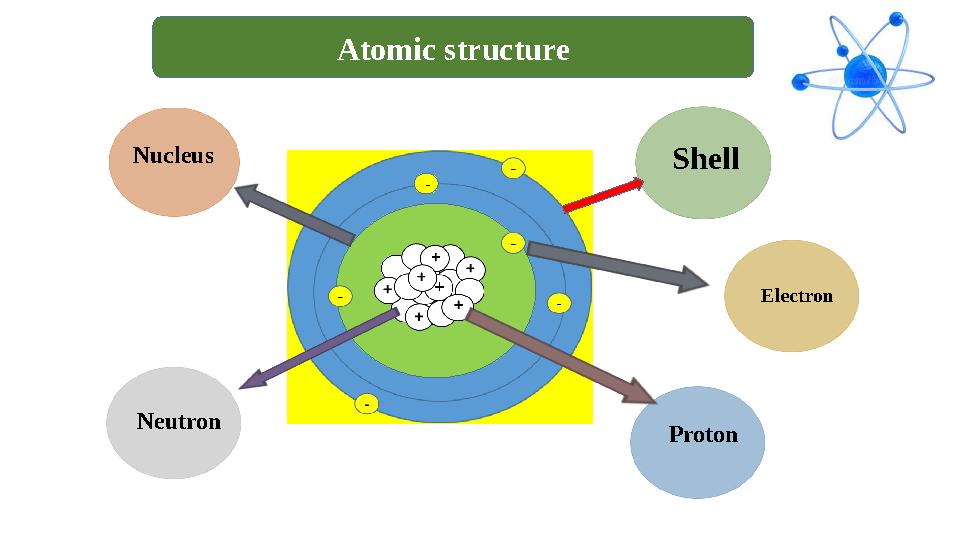
+- Shell
Electron
ProtonNeutronNucleus Atomic structure
7 слайд
+- Shell Electron ProtonNeutronNucleus Atomic structure
#8 слайд
Is located in the center of
atom.
8 слайд
Is located in the center of atom.
#9 слайд
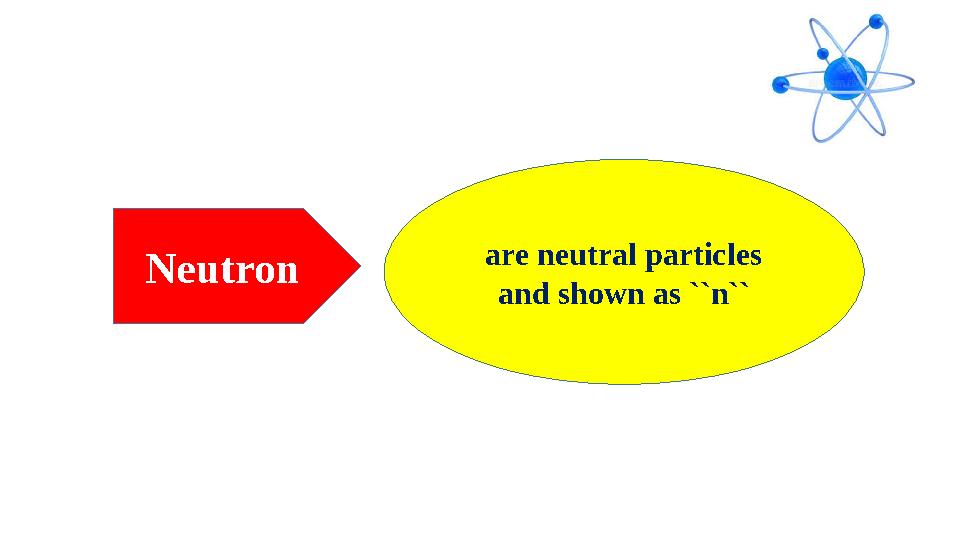
Neutron are neutral particles
and shown as ``n``
9 слайд
Neutron are neutral particles and shown as ``n``
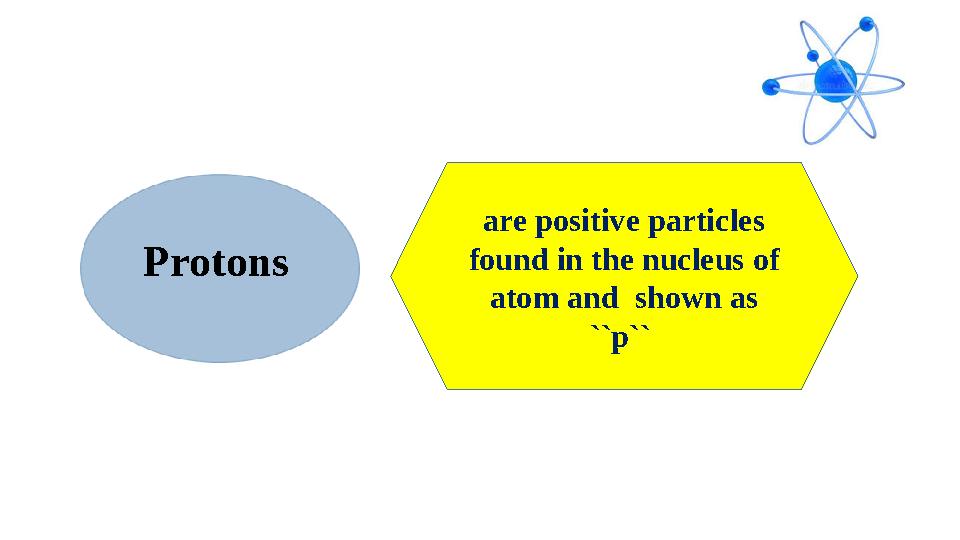
#10 слайд
Protons are positive particles
found in the nucleus of
atom and shown as
``p``
10 слайд
Protons are positive particles found in the nucleus of atom and shown as ``p``
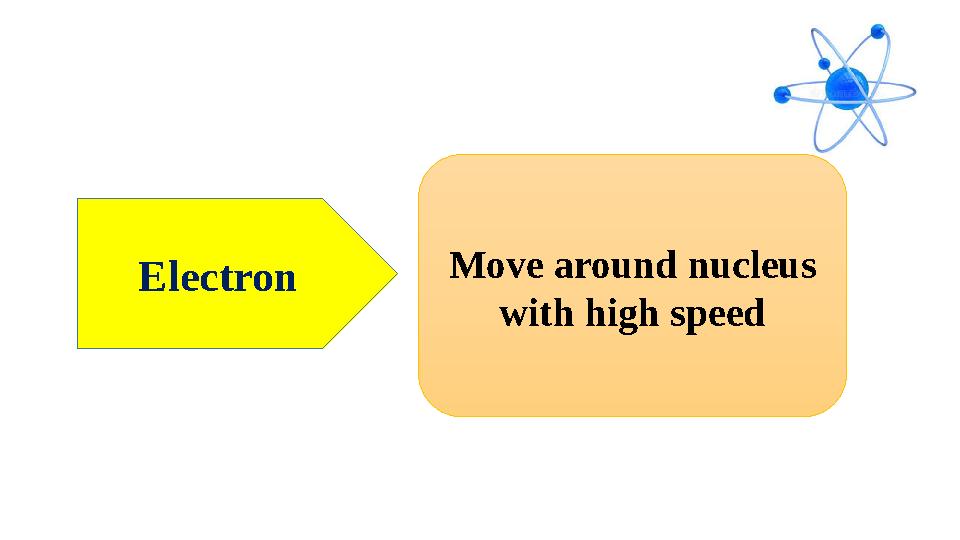
#11 слайд
Electron Move around nucleus
with high speed
11 слайд
Electron Move around nucleus with high speed
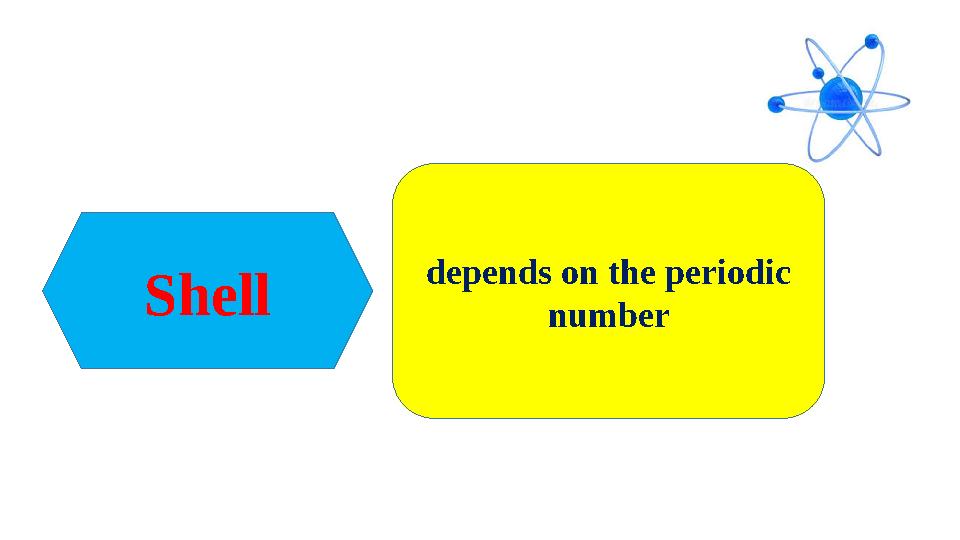
#12 слайд
Shell depends on the periodic
number
12 слайд
Shell depends on the periodic number
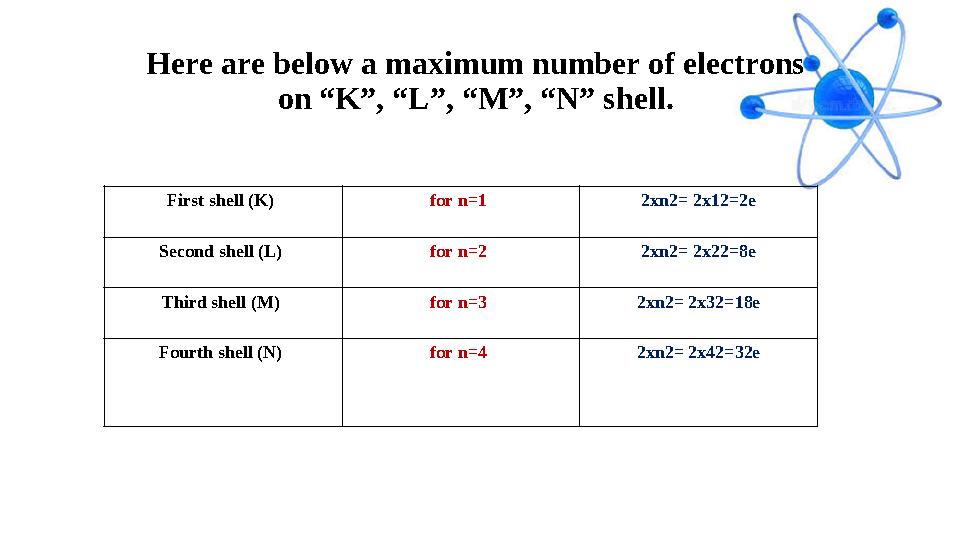
#13 слайд
Here are below a maximum number of electrons
on “K”, “L”, “M”, “N” shell.
First shell (K) for n=1 2xn2= 2x12=2e
Second shell (L) for n=2 2xn2= 2x22=8e
Third shell (M) for n=3 2xn2= 2x32=18e
Fourth shell (N) for n=4 2xn2= 2x42=32e
13 слайд
Here are below a maximum number of electrons on “K”, “L”, “M”, “N” shell. First shell (K) for n=1 2xn2= 2x12=2e Second shell (L) for n=2 2xn2= 2x22=8e Third shell (M) for n=3 2xn2= 2x32=18e Fourth shell (N) for n=4 2xn2= 2x42=32e
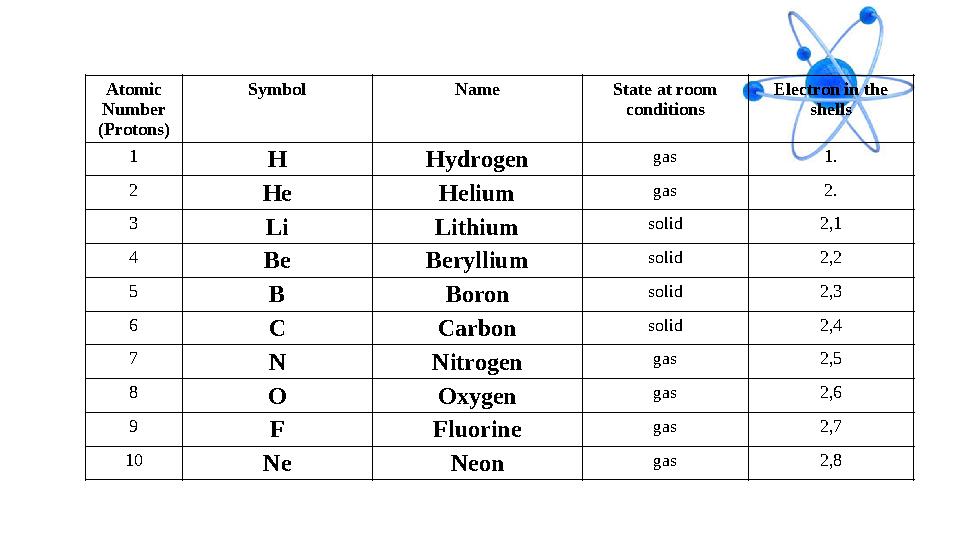
#14 слайд
Atomic
Number
(Protons) Symbol Name State at room
conditions Electron in the
shells
1
H Hydrogen gas 1.
2
He Helium gas 2.
3
Li Lithium solid 2,1
4
Be Beryllium solid 2,2
5
B Boron solid 2,3
6
C Carbon solid 2,4
7
N Nitrogen gas 2,5
8
O Oxygen gas 2,6
9
F Fluorine gas 2,7
10
Ne Neon gas 2,8
14 слайд
Atomic Number (Protons) Symbol Name State at room conditions Electron in the shells 1 H Hydrogen gas 1. 2 He Helium gas 2. 3 Li Lithium solid 2,1 4 Be Beryllium solid 2,2 5 B Boron solid 2,3 6 C Carbon solid 2,4 7 N Nitrogen gas 2,5 8 O Oxygen gas 2,6 9 F Fluorine gas 2,7 10 Ne Neon gas 2,8
#15 слайд
General description
of element
15 слайд
General description of element

#16 слайд
16 слайд
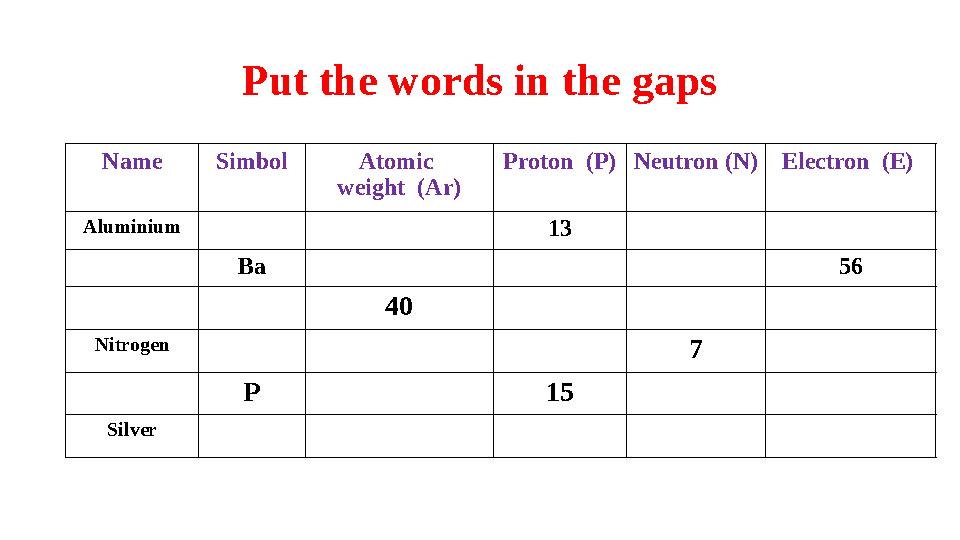
#17 слайд
Put the words in the gaps
Name Simbol Atomic
weight (Ar) Proton (P) Neutron (N) Electron (E)
Aluminium
13
Ba 56
40
Nitrogen
7
P 15
Silver
17 слайд
Put the words in the gaps Name Simbol Atomic weight (Ar) Proton (P) Neutron (N) Electron (E) Aluminium 13 Ba 56 40 Nitrogen 7 P 15 Silver

#18 слайд
Nucleus shell, atom neutral, negative electrons orbital,
positive protons, neutron
Scientists say that the smallest particle of substances are called _____ . Atom means indivisible derived from
atomos in Greek language because of its very small size. Atom has two parts, nucleus and _______ . _________ is
located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed that atoms were
indivisible up to 20 th
century. Today we know that atoms have subatomic particles, called _______ , ________ and
electrons.
Protons are _______ particles found in the nucleus of an atom, and shown as « p » . Each element has certain
number of protons which differ from other elements. Neutrons are _________ particles found in the nucleus of an atom,
and shown as « n » . Electrons are _______ particles that move around the nucleus of an atom, and shown as “e”. Neutral
atoms have the equal number of protons and electrons. Electrons are rotating in certain places called _________ energy
level or shell. The electrons located in the outermost shell of atoms are called _____________ . __________ have the
same number of protons but different number of neutrons. They have similar chemical properties but different physical
properties.
18 слайд
Nucleus shell, atom neutral, negative electrons orbital, positive protons, neutron Scientists say that the smallest particle of substances are called _____ . Atom means indivisible derived from atomos in Greek language because of its very small size. Atom has two parts, nucleus and _______ . _________ is located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed that atoms were indivisible up to 20 th century. Today we know that atoms have subatomic particles, called _______ , ________ and electrons. Protons are _______ particles found in the nucleus of an atom, and shown as « p » . Each element has certain number of protons which differ from other elements. Neutrons are _________ particles found in the nucleus of an atom, and shown as « n » . Electrons are _______ particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called _________ energy level or shell. The electrons located in the outermost shell of atoms are called _____________ . __________ have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.
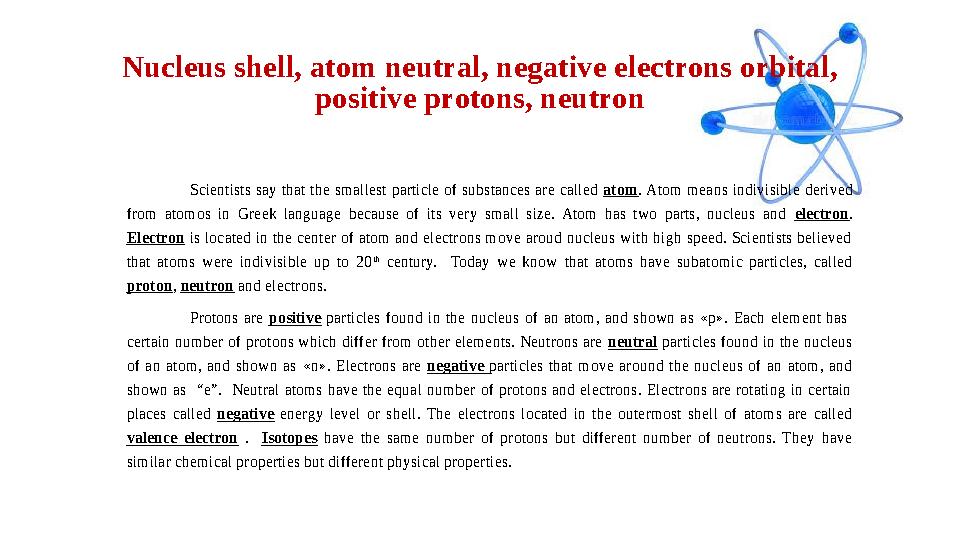
#19 слайд
Nucleus shell, atom neutral, negative electrons orbital,
positive protons, neutron
Scientists say that the smallest particle of substances are called atom . Atom means indivisible derived
from atomos in Greek language because of its very small size. Atom has two parts, nucleus and electron .
Electron is located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed
that atoms were indivisible up to 20 th
century. Today we know that atoms have subatomic particles, called
proton , neutron and electrons.
Protons are positive particles found in the nucleus of an atom, and shown as « p » . Each element has
certain number of protons which differ from other elements. Neutrons are neutral particles found in the nucleus
of an atom, and shown as « n » . Electrons are negative particles that move around the nucleus of an atom, and
shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain
places called negative energy level or shell. The electrons located in the outermost shell of atoms are called
valence electron . Isotopes have the same number of protons but different number of neutrons. They have
similar chemical properties but different physical properties.
19 слайд
Nucleus shell, atom neutral, negative electrons orbital, positive protons, neutron Scientists say that the smallest particle of substances are called atom . Atom means indivisible derived from atomos in Greek language because of its very small size. Atom has two parts, nucleus and electron . Electron is located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed that atoms were indivisible up to 20 th century. Today we know that atoms have subatomic particles, called proton , neutron and electrons. Protons are positive particles found in the nucleus of an atom, and shown as « p » . Each element has certain number of protons which differ from other elements. Neutrons are neutral particles found in the nucleus of an atom, and shown as « n » . Electrons are negative particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called negative energy level or shell. The electrons located in the outermost shell of atoms are called valence electron . Isotopes have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.

#20 слайд
Complete the sentences and pronounce them
•
Atom is _____________
•
From Greek language atomos means _____________
•
Atom has two parts: ______ and _______
•
Protons are _____________ and shown as `` p``
•
Neutrons are _________ and shown as ``n``
•
Electrons are _________ and shown as ``e``
•
Isotopes have ___________________ .
20 слайд
Complete the sentences and pronounce them • Atom is _____________ • From Greek language atomos means _____________ • Atom has two parts: ______ and _______ • Protons are _____________ and shown as `` p`` • Neutrons are _________ and shown as ``n`` • Electrons are _________ and shown as ``e`` • Isotopes have ___________________ .

#21 слайд
Complete the sentences and pronounce them
•
Atom is the smallest particle of substances
•
From Greek language atomos means very small size
•
Atom has two parts: nucleus and electron
•
Protons are positive particles found in the nucleus of atom and shown as ``
p``
•
Neutrons are neutral particles and shown as ``n``
•
Electrons are negative particles than move around the nucleus of on atom
and shown and shown as ``e``
•
Isotopes have the some number of protons but different number of
neutrons .
21 слайд
Complete the sentences and pronounce them • Atom is the smallest particle of substances • From Greek language atomos means very small size • Atom has two parts: nucleus and electron • Protons are positive particles found in the nucleus of atom and shown as `` p`` • Neutrons are neutral particles and shown as ``n`` • Electrons are negative particles than move around the nucleus of on atom and shown and shown as ``e`` • Isotopes have the some number of protons but different number of neutrons .

#22 слайд
Постер қорғау
22 слайд
Постер қорғау

#23 слайд
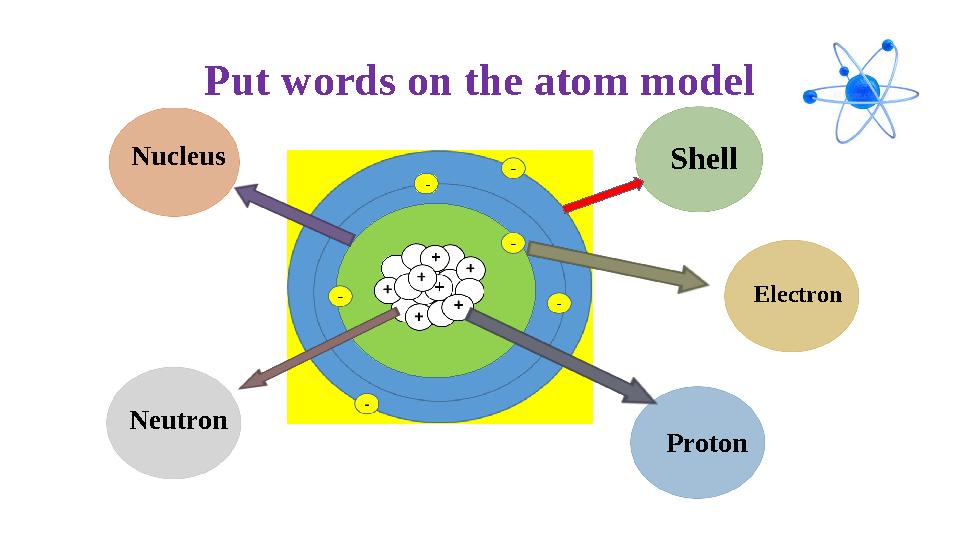
Put words on the atom model
+-
23 слайд
Put words on the atom model +-
#24 слайд
Put words on the atom model
+- Shell
Electron
ProtonNeutron Nucleus
24 слайд
Put words on the atom model +- Shell Electron ProtonNeutron Nucleus

#25 слайд
Work in pairs. Ask the following question
to a partner
•
What is atom?
•
What is the meaning of atomos from Greek language?
•
What are the two parts that atom has?
•
What is definition of protons?
•
What is definition of neutrons?
•
What is definition of electrons?
•
What is definition of isotope?
25 слайд
Work in pairs. Ask the following question to a partner • What is atom? • What is the meaning of atomos from Greek language? • What are the two parts that atom has? • What is definition of protons? • What is definition of neutrons? • What is definition of electrons? • What is definition of isotope?

шағым қалдыра аласыз