Ерітінділер
Ерітінділер


#1 слайд
SOLUTIONS
SOLUBILITY
1 слайд
SOLUTIONS SOLUBILITY

#2 слайд
Plan of the lesson:
You will
Terminology
STQ
New topic
Learning check
2 слайд
Plan of the lesson: You will Terminology STQ New topic Learning check

#3 слайд
You will:
●
classify the substances according to the degree of
solubility;
●
learn about solutions and their importance;
●
determine the composition of salt solutions by
using evaporation method;
●
know and determine saturated solutions.
3 слайд
You will: ● classify the substances according to the degree of solubility; ● learn about solutions and their importance; ● determine the composition of salt solutions by using evaporation method; ● know and determine saturated solutions.
#4 слайд
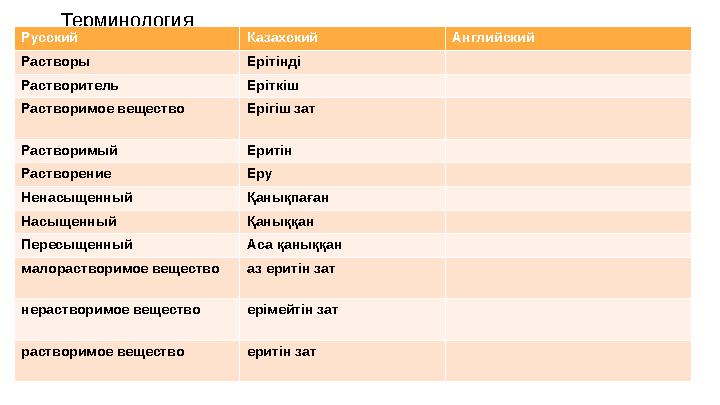
Терминология
Русский Казахский Английский
Растворы Ерітінді
Растворитель Еріткіш
Растворимое вещество Ерігіш зат
Растворимый Еритін
Растворение Еру
Ненасыщенный Қанықпаған
Насыщенный Қаныққан
Пересыщенный Аса қаныққан
малорастворимое вещество аз еритін зат
нерастворимое вещество ерімейтін зат
растворимое вещество еритін зат
4 слайд
Терминология Русский Казахский Английский Растворы Ерітінді Растворитель Еріткіш Растворимое вещество Ерігіш зат Растворимый Еритін Растворение Еру Ненасыщенный Қанықпаған Насыщенный Қаныққан Пересыщенный Аса қаныққан малорастворимое вещество аз еритін зат нерастворимое вещество ерімейтін зат растворимое вещество еритін зат
#5 слайд
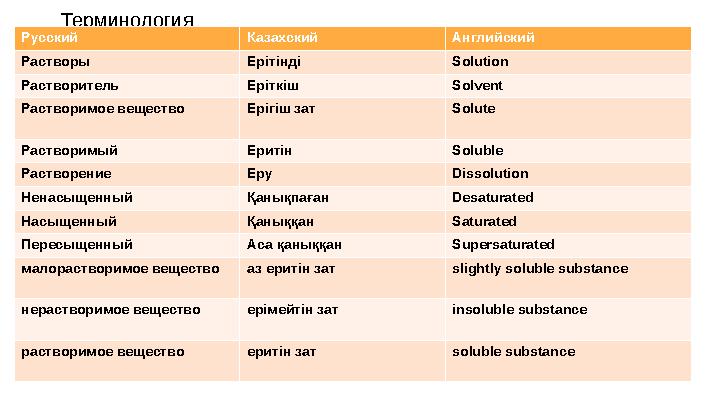
Терминология
Русский Казахский Английский
Растворы Ерітінді Solution
Растворитель Еріткіш Solvent
Растворимое вещество Ерігіш зат Solute
Растворимый Еритін Soluble
Растворение Еру Dissolution
Ненасыщенный Қанықпаған Desaturated
Насыщенный Қаныққан Saturated
Пересыщенный Аса қаныққан Supersaturated
малорастворимое вещество аз еритін зат slightly soluble substance
нерастворимое вещество ерімейтін зат insoluble substance
растворимое вещество еритін зат soluble substance
5 слайд
Терминология Русский Казахский Английский Растворы Ерітінді Solution Растворитель Еріткіш Solvent Растворимое вещество Ерігіш зат Solute Растворимый Еритін Soluble Растворение Еру Dissolution Ненасыщенный Қанықпаған Desaturated Насыщенный Қаныққан Saturated Пересыщенный Аса қаныққан Supersaturated малорастворимое вещество аз еритін зат slightly soluble substance нерастворимое вещество ерімейтін зат insoluble substance растворимое вещество еритін зат soluble substance
#6 слайд
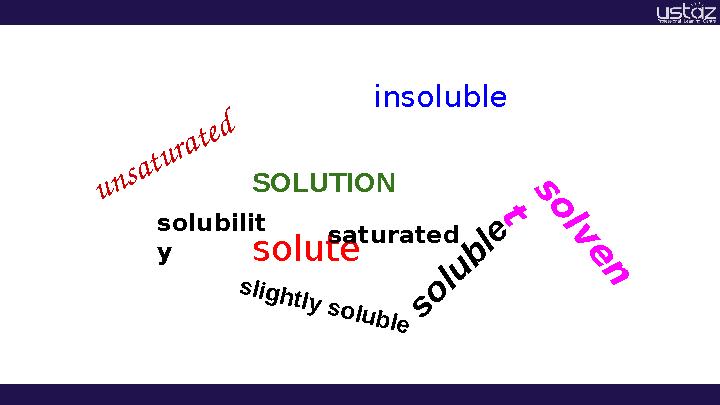
SOLUTION
solubilit
y
solutes
o
lu
b
le
s lig h tly s o lu b le insoluble
s
o
lv
e
n
t
saturated
u n sa t u ra t e d
6 слайд
SOLUTION solubilit y solutes o lu b le s lig h tly s o lu b le insoluble s o lv e n t saturated u n sa t u ra t e d

#7 слайд
Why is sea water salty?
Why is sugar more soluble in
water than salt?
7 слайд
Why is sea water salty? Why is sugar more soluble in water than salt?

#8 слайд
Some substances
dissolve in water e.g.
salt white solid
colourless
solution
8 слайд
Some substances dissolve in water e.g. salt white solid colourless solution

#9 слайд
Some substances
dissolve in water e.g.
copper sulfate coloured solid
coloured solution
9 слайд
Some substances dissolve in water e.g. copper sulfate coloured solid coloured solution

#10 слайд
Some substances
dissolve in water e.g.
nickel sulfate coloured solid
coloured solution
10 слайд
Some substances dissolve in water e.g. nickel sulfate coloured solid coloured solution

#11 слайд
SOLUBLE
Dissolves
(e.g. salt in water) INSOLUBLE
Does not dissolve
(e.g. sand in water)
11 слайд
SOLUBLE Dissolves (e.g. salt in water) INSOLUBLE Does not dissolve (e.g. sand in water)

#12 слайд
Is each substance
SOLUBLE or INSOLUBLE in water?
sugar flour
gloss paint
sand
glass salt
12 слайд
Is each substance SOLUBLE or INSOLUBLE in water? sugar flour gloss paint sand glass salt

#13 слайд
When a substance dissolves:
•
the solid “disappears”
•
the solution is transparent
•
the solution is coloured (if a coloured solid) or
colourless (if a white solid)
When a substance does NOT dissolve:
•
the solid is still visible in a colourless liquid
or
•
the liquid is cloudy
13 слайд
When a substance dissolves: • the solid “disappears” • the solution is transparent • the solution is coloured (if a coloured solid) or colourless (if a white solid) When a substance does NOT dissolve: • the solid is still visible in a colourless liquid or • the liquid is cloudy

#14 слайд
SOLUTE
Substance
dissolving SOLUTION
Solute dissolved
in solventSOLVENT
Liquid the solute
dissolves in
14 слайд
SOLUTE Substance dissolving SOLUTION Solute dissolved in solventSOLVENT Liquid the solute dissolves in

#15 слайд
There are many solvents:
e.g. water
Saline solution
(salt dissolved in water)
15 слайд
There are many solvents: e.g. water Saline solution (salt dissolved in water)

#16 слайд
There are many solvents:
e.g. ethanol
Iodine tincture
(iodine dissolved in
ethanol)
used as an antiseptic
16 слайд
There are many solvents: e.g. ethanol Iodine tincture (iodine dissolved in ethanol) used as an antiseptic

#17 слайд
Used to dissolve dirt in
dry cleaningThere are many solvents:
e.g. perchloroethylene
17 слайд
Used to dissolve dirt in dry cleaningThere are many solvents: e.g. perchloroethylene
#18 слайд
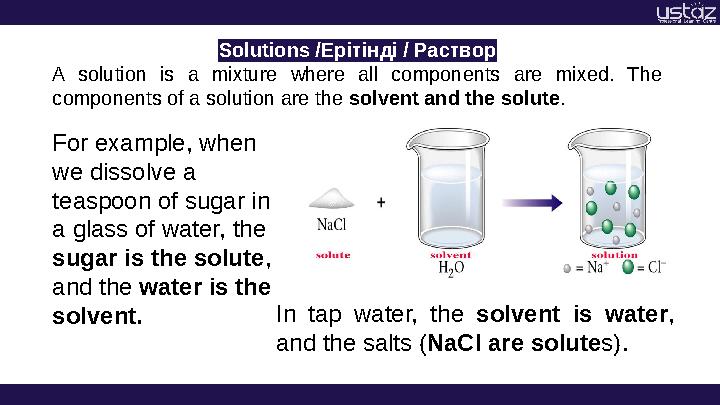
Solutions /Ерітінді / Раствор
A solution is a mixture where all components are mixed. The
components of a solution are the solvent and the solute .
For example, when
we dissolve a
teaspoon of sugar in
a glass of water, the
sugar is the solute ,
and the water is the
solvent. In tap water, the solvent is water ,
and the salts ( NaCl are solute s).
18 слайд
Solutions /Ерітінді / Раствор A solution is a mixture where all components are mixed. The components of a solution are the solvent and the solute . For example, when we dissolve a teaspoon of sugar in a glass of water, the sugar is the solute , and the water is the solvent. In tap water, the solvent is water , and the salts ( NaCl are solute s).

#19 слайд
TYPES OF SOLUTIONS
State of solvent State of solute State of solution Examples
Gas Gas Gas Air, Natural gas
Liquid Liquid Liquid Alcoholic beverages,
Antifreeze solution
Liquid Solid Liquid Seawater, Sugar solution
Liquid Gas Liquid Carbonated water(soda),
Ammonia solution
Solid Solid Solid Metal alloys: brass and
bronze
Solid Gas Solid Hydrogen in platinum
19 слайд
TYPES OF SOLUTIONS State of solvent State of solute State of solution Examples Gas Gas Gas Air, Natural gas Liquid Liquid Liquid Alcoholic beverages, Antifreeze solution Liquid Solid Liquid Seawater, Sugar solution Liquid Gas Liquid Carbonated water(soda), Ammonia solution Solid Solid Solid Metal alloys: brass and bronze Solid Gas Solid Hydrogen in platinum

#20 слайд
Solubility / Ерігіштік / Растворимость
The solubility of a compound is the maximum amount of solute dissolving in
a given solvent to form a saturated solution. Solubility data are reported in
units of grams of solute per 100 g of water. Each solid has a different
solubility in water.
The solubility of table salt NaCl is 36
g in 100 g of water at 20 o
C The solubility of sugar is 204g in 100
g of water at 20 o
C.
20 слайд
Solubility / Ерігіштік / Растворимость The solubility of a compound is the maximum amount of solute dissolving in a given solvent to form a saturated solution. Solubility data are reported in units of grams of solute per 100 g of water. Each solid has a different solubility in water. The solubility of table salt NaCl is 36 g in 100 g of water at 20 o C The solubility of sugar is 204g in 100 g of water at 20 o C.

#21 слайд
21 слайд
#22 слайд
Learning check
Identify the solute in each of the following solutions:
1. 2 g of sugar (A) and 100 mL of water (B)
2. 60.0 ml of ethyl alcohol (A) and 30.0 mL of methyl alcohol (B)
3. 55.0 ml of water (A) and 1,50 g of NaCl (B)
4. Air: 200 ml of O
2 (A) and 800mL of N
2 (B)
22 слайд
Learning check Identify the solute in each of the following solutions: 1. 2 g of sugar (A) and 100 mL of water (B) 2. 60.0 ml of ethyl alcohol (A) and 30.0 mL of methyl alcohol (B) 3. 55.0 ml of water (A) and 1,50 g of NaCl (B) 4. Air: 200 ml of O 2 (A) and 800mL of N 2 (B)

шағым қалдыра аласыз