Презентация Периодтық кестенің құрылымы
Презентация Периодтық кестенің құрылымы

#1 слайд
Teacher name :
Zhangbyrbay Moldir
1 слайд
Teacher name : Zhangbyrbay Moldir
#2 слайд
Structure of Structure of
Periodic Periodic
TableTable
2 слайд
Structure of Structure of Periodic Periodic TableTable
#3 слайд
8.2.1.1. Explain the PT atomic 8.2.1.1. Explain the PT atomic
number`s physical meaning number`s physical meaning
Interactive open lesson with elements Interactive open lesson with elements
of the English languageof the English language
1. All learners will be able to describe the 1. All learners will be able to describe the
structure of the PT by use of terms as group structure of the PT by use of terms as group
and know that Chemistry period number and and know that Chemistry period number and
can make some diagramcan make some diagram
;;
2.All learners know that PT has got some 2.All learners know that PT has got some
important numbers as atomic number and some important numbers as atomic number and some
number of group and periodnumber of group and period
;;Structure of Periodic Structure of Periodic
TableTable
3 слайд
8.2.1.1. Explain the PT atomic 8.2.1.1. Explain the PT atomic number`s physical meaning number`s physical meaning Interactive open lesson with elements Interactive open lesson with elements of the English languageof the English language 1. All learners will be able to describe the 1. All learners will be able to describe the structure of the PT by use of terms as group structure of the PT by use of terms as group and know that Chemistry period number and and know that Chemistry period number and can make some diagramcan make some diagram ;; 2.All learners know that PT has got some 2.All learners know that PT has got some important numbers as atomic number and some important numbers as atomic number and some number of group and periodnumber of group and period ;;Structure of Periodic Structure of Periodic TableTable
#4 слайд
4 слайд

#5 слайд
№№
1 Exercise1 Exercise
Working with textWorking with text
5 слайд
№№ 1 Exercise1 Exercise Working with textWorking with text

#6 слайд
№№
2 Exercise2 Exercise
Chemical dictation Chemical dictation
The modern periodic table contains all know
elements. It shows us physical and chemical
properties elements. A simple PT contains the
symbols , atomic numbers , and the relative of the
elements. Each horizontal row in the PT is called a
period . There are seven periods in the modern PT
and each period begins with alkali metal and end
with a noble gas . However, the first element of the
first period hydrogen is not a metal . Each
vertical column in the PT is called a group . There
are eighteen groups in the PT . The number of
electrons in the outer shell is the number of a
group .
6 слайд
№№ 2 Exercise2 Exercise Chemical dictation Chemical dictation The modern periodic table contains all know elements. It shows us physical and chemical properties elements. A simple PT contains the symbols , atomic numbers , and the relative of the elements. Each horizontal row in the PT is called a period . There are seven periods in the modern PT and each period begins with alkali metal and end with a noble gas . However, the first element of the first period hydrogen is not a metal . Each vertical column in the PT is called a group . There are eighteen groups in the PT . The number of electrons in the outer shell is the number of a group .

#7 слайд
3-Ex3-Ex
ee
rcisercise
Find element which are Find element which are
named after named after
1- Group - Scientists1- Group - Scientists
2- Group - Planets2- Group - Planets
3- Group - Geographical 3- Group - Geographical
placesplaces
7 слайд
3-Ex3-Ex ee rcisercise Find element which are Find element which are named after named after 1- Group - Scientists1- Group - Scientists 2- Group - Planets2- Group - Planets 3- Group - Geographical 3- Group - Geographical placesplaces
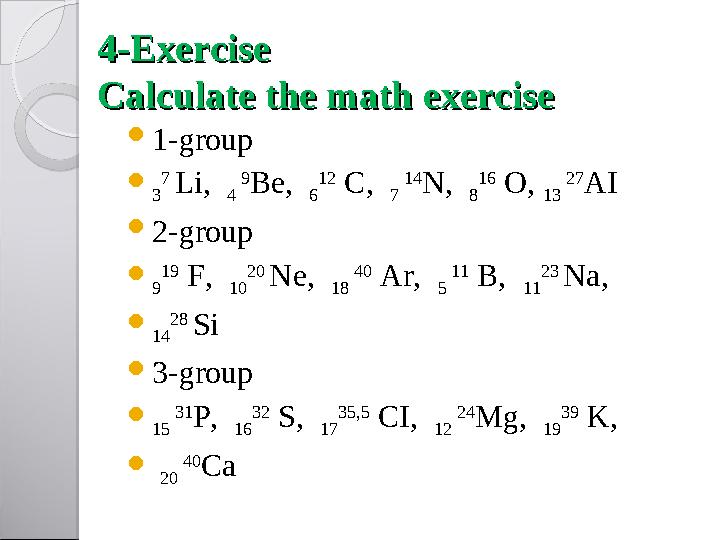
#8 слайд
44
-Ex-Ex
ee
rcisercise
Calculate the math exercise Calculate the math exercise
1-group
3 7
Li,
4 9
Be,
6 12
C,
7 14
N,
8 16
O,
13 27
AI
2-group
9 19
F,
10 20
Ne,
18 40
Ar,
5 11
B,
11 23
Na,
14 28
Si
3-group
15 31
P,
16 32
S,
17 35,5
CI,
12 24
Mg,
19 39
K,
20 40
Са
8 слайд
44 -Ex-Ex ee rcisercise Calculate the math exercise Calculate the math exercise 1-group 3 7 Li, 4 9 Be, 6 12 C, 7 14 N, 8 16 O, 13 27 AI 2-group 9 19 F, 10 20 Ne, 18 40 Ar, 5 11 B, 11 23 Na, 14 28 Si 3-group 15 31 P, 16 32 S, 17 35,5 CI, 12 24 Mg, 19 39 K, 20 40 Са
#9 слайд
5-Exercise5-Exercise
Thinking – Couple – Share Thinking – Couple – Share
9 слайд
5-Exercise5-Exercise Thinking – Couple – Share Thinking – Couple – Share
#10 слайд
6-Exercise6-Exercise
Working with empty table of the PTWorking with empty table of the PT
10 слайд
6-Exercise6-Exercise Working with empty table of the PTWorking with empty table of the PT
#11 слайд
Work with the term Work with the term
11 слайд
Work with the term Work with the term
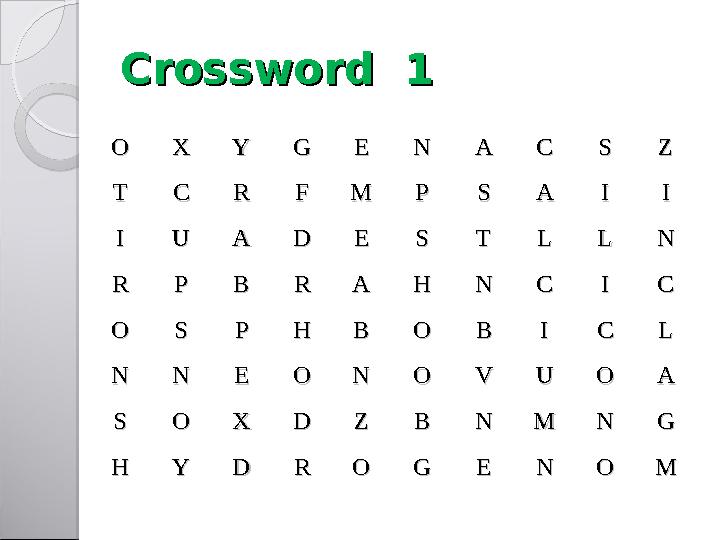
#12 слайд
Crossword 1Crossword 1
OO
XX
YY
GG
EE
NN
AA
CC
SS
ZZ
TT
CC
RR
FF
MM
PP
SS
AA
II
II
II
UU
AA
DD
EE
SS
TT
LL
LL
NN
RR
PP
BB
RR
AA
HH
NN
CC
II
CC
OO
SS
PP
HH
BB
OO
BB
II
CC
LL
NN
NN
EE
OO
NN
OO
VV
UU
OO
AA
SS
OO
XX
DD
ZZ
BB
NN
MM
NN
GG
HH
YY
DD
RR
OO
GG
EE
NN
OO
MM
12 слайд
Crossword 1Crossword 1 OO XX YY GG EE NN AA CC SS ZZ TT CC RR FF MM PP SS AA II II II UU AA DD EE SS TT LL LL NN RR PP BB RR AA HH NN CC II CC OO SS PP HH BB OO BB II CC LL NN NN EE OO NN OO VV UU OO AA SS OO XX DD ZZ BB NN MM NN GG HH YY DD RR OO GG EE NN OO MM
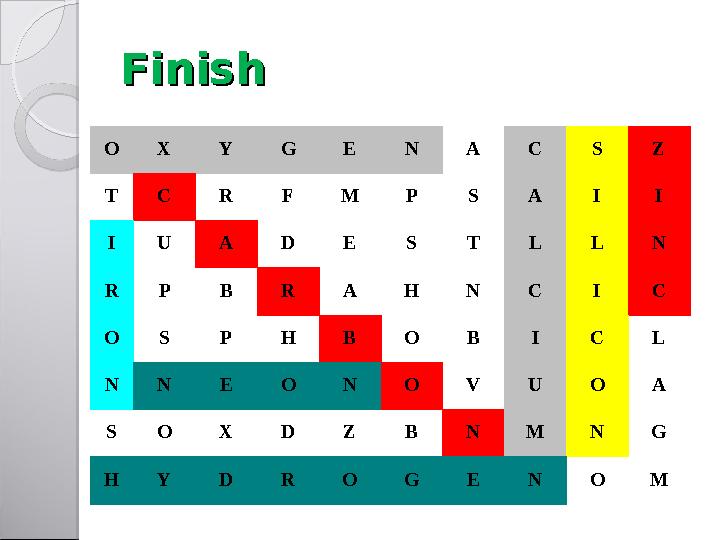
#13 слайд
FinishFinish
O X Y G E N A C S Z
T C R F M P S A I I
I U A D E S T L L N
R P B R A H N C I C
O S P H B O B I C L
N N E O N O V U O A
S O X D Z B N M N G
H Y D R O G E N O M
13 слайд
FinishFinish O X Y G E N A C S Z T C R F M P S A I I I U A D E S T L L N R P B R A H N C I C O S P H B O B I C L N N E O N O V U O A S O X D Z B N M N G H Y D R O G E N O M
#14 слайд
Crossword 2Crossword 2
This element has atomic number of 6
It has 1 proton
This element has atomic mass 35,5
This element’s number is 12
This element’s is 4 period 2 group B
This element has atomic mass of 32
It has 7 electrons
This element has symbol of Cu
This element has 14 protons
14 слайд
Crossword 2Crossword 2 This element has atomic number of 6 It has 1 proton This element has atomic mass 35,5 This element’s number is 12 This element’s is 4 period 2 group B This element has atomic mass of 32 It has 7 electrons This element has symbol of Cu This element has 14 protons

#15 слайд
FinishFinish
1 C A R B O N
2 H Y D R O G E N
3 C H L O R I N E
4 M A G N E S I U M
5 Z I N C
6 S U L F U R
7 N I T R O G E N
8 C O P P E R
9 S I L I C O N
15 слайд
FinishFinish 1 C A R B O N 2 H Y D R O G E N 3 C H L O R I N E 4 M A G N E S I U M 5 Z I N C 6 S U L F U R 7 N I T R O G E N 8 C O P P E R 9 S I L I C O N

#16 слайд
ReflectionReflection
16 слайд
ReflectionReflection

#17 слайд
HOME WORKHOME WORK
Our homework is to memorizeOur homework is to memorize
the new wordsthe new words
17 слайд
HOME WORKHOME WORK Our homework is to memorizeOur homework is to memorize the new wordsthe new words

#18 слайд
18 слайд

#19 слайд
19 слайд

#20 слайд
20 слайд

шағым қалдыра аласыз