Trends in Periodic table
Trends in Periodic table

#1 слайд
Trends in Periodic table
•
You will :
•
- know how are properties of elements
changed among groups and periods
•
- be able to predict properties of an element by
the place in Periodic Table
1 слайд
Trends in Periodic table • You will : • - know how are properties of elements changed among groups and periods • - be able to predict properties of an element by the place in Periodic Table

#2 слайд
Plan of the lesson :
•
- Greeting
•
-Home task
•
-Terminology
•
-New topic
•
-Cheeking understanding
2 слайд
Plan of the lesson : • - Greeting • -Home task • -Terminology • -New topic • -Cheeking understanding

#3 слайд
Work in pairs and answer the following questions
1. When and by whom the periodic table was opened?
2. How are horizontal rows called?
3.What is the name of vertical columns in a periodic table ?
4. What is the number of periods in a periodic table ?
5. How many columns are there in periodic table ?
6. How are « A » groups called?
7. How are « B » groups called?
8.What are the exampls of alkali metals?
9. What are the exampls of noble gases?
10. Write electron configuration of : 3Li 7N 11 Na
3 слайд
Work in pairs and answer the following questions 1. When and by whom the periodic table was opened? 2. How are horizontal rows called? 3.What is the name of vertical columns in a periodic table ? 4. What is the number of periods in a periodic table ? 5. How many columns are there in periodic table ? 6. How are « A » groups called? 7. How are « B » groups called? 8.What are the exampls of alkali metals? 9. What are the exampls of noble gases? 10. Write electron configuration of : 3Li 7N 11 Na
#4 слайд
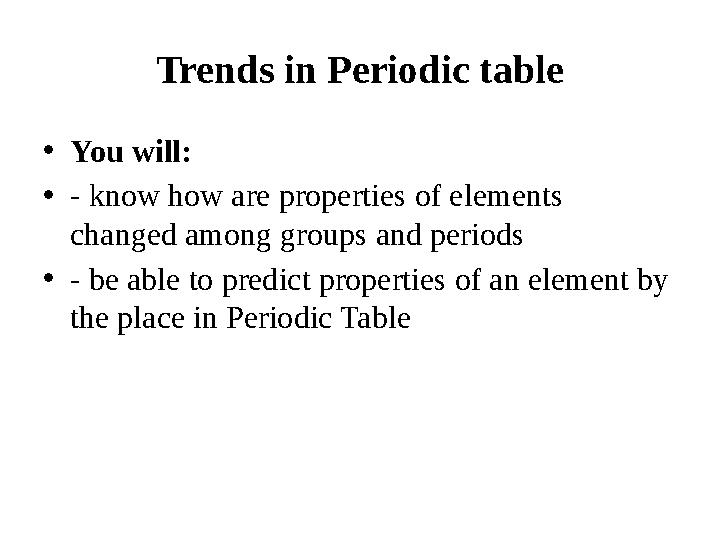
Terminology
to predict предсказывать болжау
Properties свойства қасиеттері
Electronegativity электроотрицательность электртерістік
Paulings scale Шкала Полинга Полинг
шкаласы
Value значение шама
Located расположенный орналасқан
to assume представлять елестету
4 слайд
Terminology to predict предсказывать болжау Properties свойства қасиеттері Electronegativity электроотрицательность электртерістік Paulings scale Шкала Полинга Полинг шкаласы Value значение шама Located расположенный орналасқан to assume представлять елестету

#5 слайд
Is it possible to predict properties of an
element just by looking at the Periodic
Table?
5 слайд
Is it possible to predict properties of an element just by looking at the Periodic Table?

#6 слайд
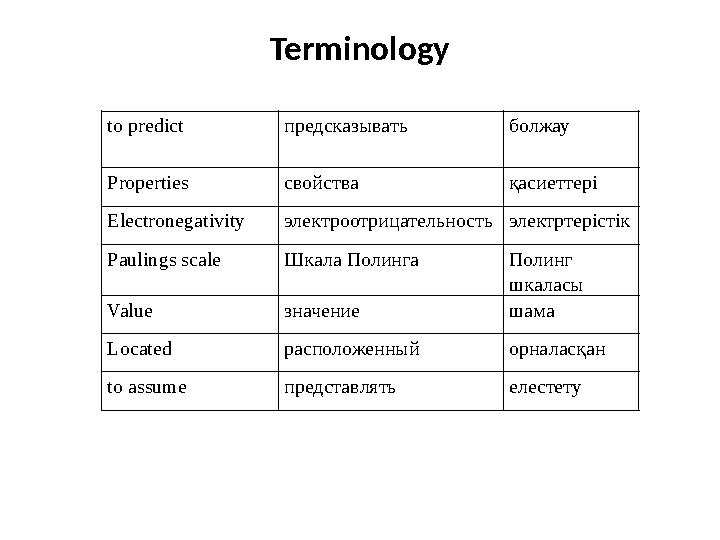
• The periodic table contains a great deal of
information packed into one location. It was
developed to illustrate recurring trends in
properties of elements. Elements in the same
vertical column have similar properties. If you
know the name of an element, the periodic
table will tell you that element’s symbol and
atomic number. With this information, you can
then learn a lot more about the make-up of
the element.
6 слайд
• The periodic table contains a great deal of information packed into one location. It was developed to illustrate recurring trends in properties of elements. Elements in the same vertical column have similar properties. If you know the name of an element, the periodic table will tell you that element’s symbol and atomic number. With this information, you can then learn a lot more about the make-up of the element.
#7 слайд
7 слайд
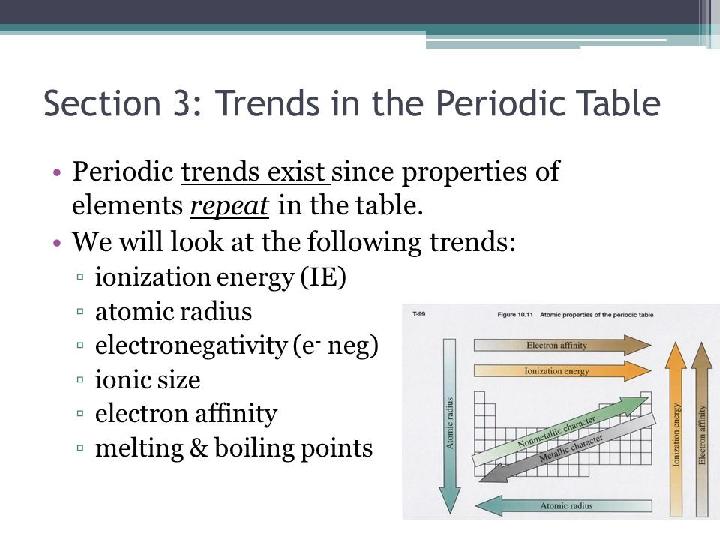
#8 слайд
8 слайд
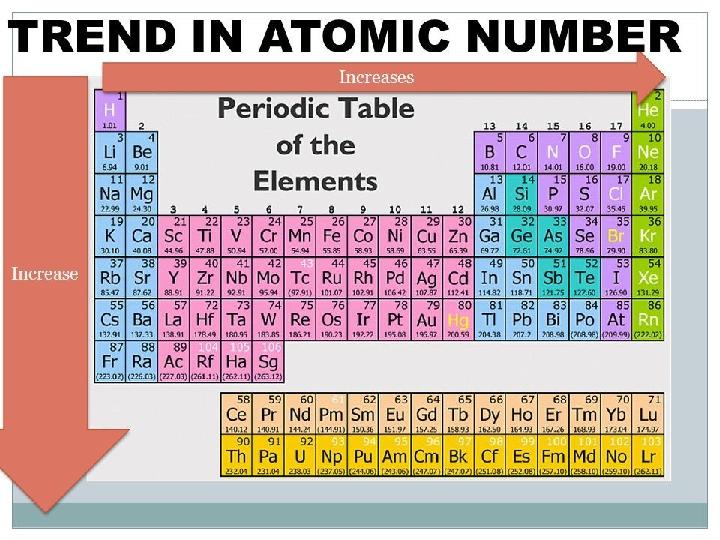
#9 слайд
9 слайд
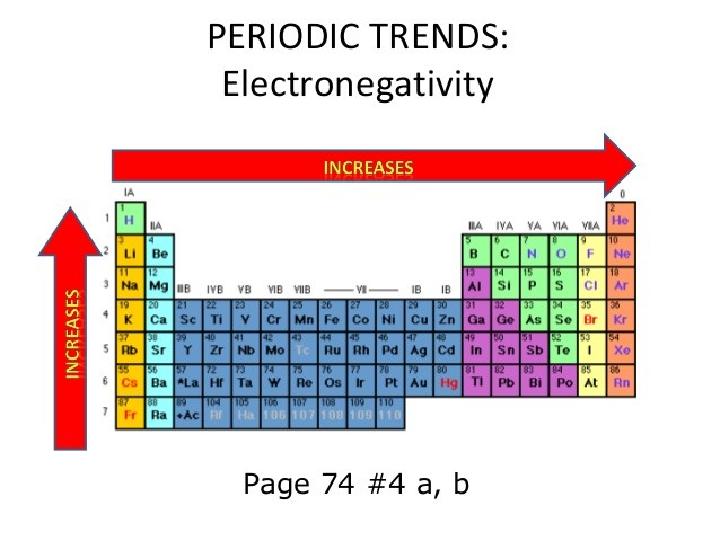
#10 слайд
10 слайд
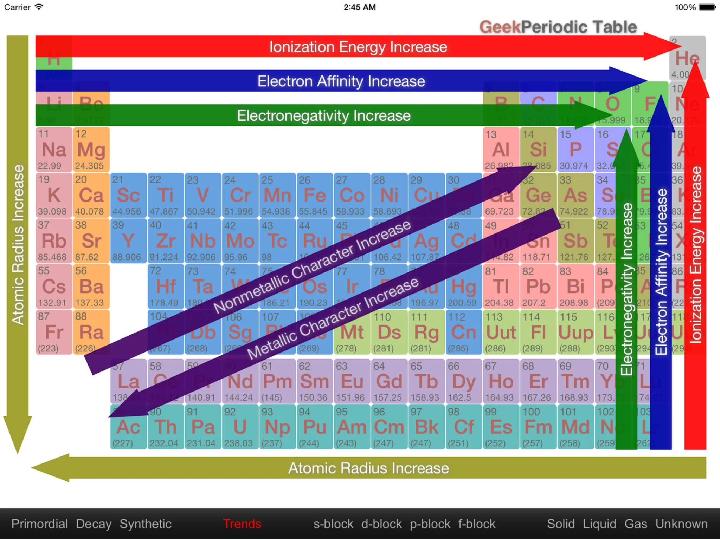
#11 слайд
11 слайд
#12 слайд
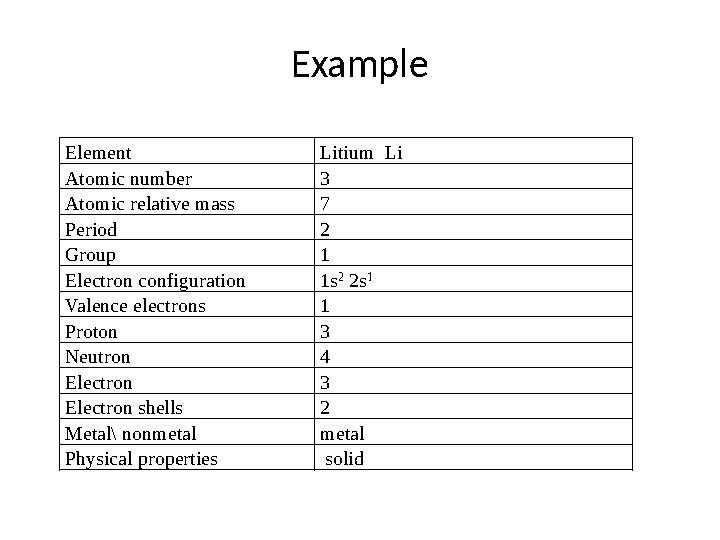
Example
Element Litium Li
Atomic number 3
Atomic relative mass 7
Period 2
Group 1
Electron configuration 1s 2
2s 1
Valence electrons 1
Proton 3
Neutron 4
Electron 3
Electron shells 2
Metal\ nonmetal metal
Physical properties solid
12 слайд
Example Element Litium Li Atomic number 3 Atomic relative mass 7 Period 2 Group 1 Electron configuration 1s 2 2s 1 Valence electrons 1 Proton 3 Neutron 4 Electron 3 Electron shells 2 Metal\ nonmetal metal Physical properties solid
#13 слайд
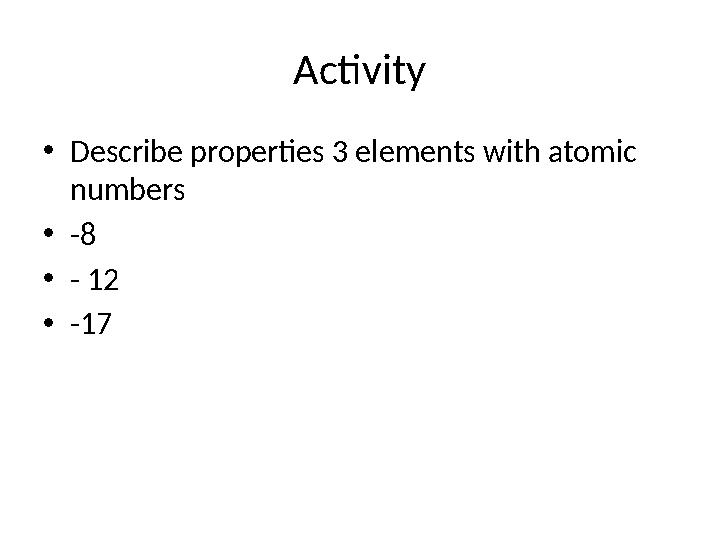
Activity
•
Describe properties 3 elements with atomic
numbers
•
-8
•
- 12
•
-17
13 слайд
Activity • Describe properties 3 elements with atomic numbers • -8 • - 12 • -17

шағым қалдыра аласыз