|
Theme of the Lesson: |
Oxides |
|||||||||||||||||||||||||
|
Subject : chemistry |
Teacher name: Ayazbaeva Gulbarshyn |
|||||||||||||||||||||||||
|
Class: 8 А |
||||||||||||||||||||||||||
|
Lesson objectives |
Give the сoncepts of Oxides and their classification, about properties |
|||||||||||||||||||||||||
|
Criteria assessment |
Learners achieve the aim if: can write classification oxides can write chemical properties |
|||||||||||||||||||||||||
|
Values |
Group work- collective, responsibility for command work result; Individual work- learn forever, honesty while doing self work |
|||||||||||||||||||||||||
|
Connection with other subjects |
Science: physics, biology |
|||||||||||||||||||||||||
|
Org moment Warm - up |
Good moorning! who is absent? who's on duty? The mossay method is divided into groups . I. "Brain Attack" 1. Types of elements? 2. The general formul of elements? 3. What is nonmetals? 4. With what do metals react? Division into the group by the Mosaic method Let is watch video |
|||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
Prezentations |
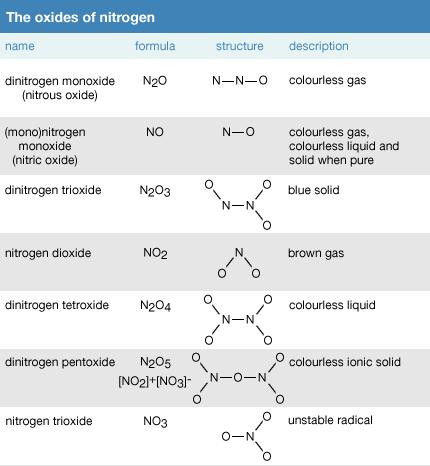
What is an Oxides?
Oxide, any of a large and important class of chemical compounds in which oxygen is combined with another element. With the exception of the lighter inert gases (helium [He], neon [Ne], argon [Ar], and krypton[Kr]), oxygen (O) forms at least one binary oxide with each of the elements.
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||
|
Practice |
grop work Lab works. 8 properties of oxides Introduction Oxides change the colours of indicators. The are corrosive.They react with metals,bases and some of the salts. Materials Sulfuric acid solution ,methylorang indicator ,piece of chalk, aluminium pieces. Procedure 1. Pour 20ml of soduim solution into the beaker,check whith methylorang indicator . 2. Add 3-4 pieces of aluminium pieces to the sulfuric acid solution . 3.Repeat procedure with adding 5 g of chalk into solution of Sulfuric acid . Observation and guestions
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
Assessment criteria |
Descriptors fully describes oxides |
|||||||||||||||||||||||||
|
end of lesson |
will respond to questions on a new topic by using the pen medium |
|||||||||||||||||||||||||
|
home work |
Oxides |
|||||||||||||||||||||||||
|
End of the lesson reflection |
writes the effect on the lessons to the smileys |
|||||||||||||||||||||||||
жүктеу мүмкіндігіне ие боласыз
Бұл материал сайт қолданушысы жариялаған. Материалдың ішінде жазылған барлық ақпаратқа жауапкершілікті жариялаған қолданушы жауап береді. Ұстаз тілегі тек ақпаратты таратуға қолдау көрсетеді. Егер материал сіздің авторлық құқығыңызды бұзған болса немесе басқа да себептермен сайттан өшіру керек деп ойласаңыз осында жазыңыз
Ашық сабақ "Oxides"
Ашық сабақ "Oxides"
|
Theme of the Lesson: |
Oxides |
|||||||||||||||||||||||||
|
Subject : chemistry |
Teacher name: Ayazbaeva Gulbarshyn |
|||||||||||||||||||||||||
|
Class: 8 А |
||||||||||||||||||||||||||
|
Lesson objectives |
Give the сoncepts of Oxides and their classification, about properties |
|||||||||||||||||||||||||
|
Criteria assessment |
Learners achieve the aim if: can write classification oxides can write chemical properties |
|||||||||||||||||||||||||
|
Values |
Group work- collective, responsibility for command work result; Individual work- learn forever, honesty while doing self work |
|||||||||||||||||||||||||
|
Connection with other subjects |
Science: physics, biology |
|||||||||||||||||||||||||
|
Org moment Warm - up |
Good moorning! who is absent? who's on duty? The mossay method is divided into groups . I. "Brain Attack" 1. Types of elements? 2. The general formul of elements? 3. What is nonmetals? 4. With what do metals react? Division into the group by the Mosaic method Let is watch video |
|||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
Prezentations |
What is an Oxides?
Oxide, any of a large and important class of chemical compounds in which oxygen is combined with another element. With the exception of the lighter inert gases (helium [He], neon [Ne], argon [Ar], and krypton[Kr]), oxygen (O) forms at least one binary oxide with each of the elements.
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||
|
Practice |
grop work Lab works. 8 properties of oxides Introduction Oxides change the colours of indicators. The are corrosive.They react with metals,bases and some of the salts. Materials Sulfuric acid solution ,methylorang indicator ,piece of chalk, aluminium pieces. Procedure 1. Pour 20ml of soduim solution into the beaker,check whith methylorang indicator . 2. Add 3-4 pieces of aluminium pieces to the sulfuric acid solution . 3.Repeat procedure with adding 5 g of chalk into solution of Sulfuric acid . Observation and guestions
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
Assessment criteria |
Descriptors fully describes oxides |
|||||||||||||||||||||||||
|
end of lesson |
will respond to questions on a new topic by using the pen medium |
|||||||||||||||||||||||||
|
home work |
Oxides |
|||||||||||||||||||||||||
|
End of the lesson reflection |
writes the effect on the lessons to the smileys |
|||||||||||||||||||||||||

шағым қалдыра аласыз