Laboratory work №8
Determination of lead in the leaves of trees growing in different areas of the city
Lesson plan:
1. determination of lead in the leaves of trees
Purpose of the work. Detection of lead in the leaves of trees growing in different areas of the city. Compare the results obtained, make predictions about the sources of pollution.
Chemical vessels and equipment:
Keldal vial
Reagents.
1 ml of sulfur and 1 ml of nitric acid
Lead
plant leaves
Brief theoretical information.
The principle of the method.
Determination of the amount of lead in plant leaves after acid treatment by atomic absorption spectrometry.
Progress of work
1.grind undigested plant material in a drying cabinet at 30-40oC and weigh 0.2 g from it.
2.The measured mass is decomposed by wet ashtray in a 300 ml Keldal vial. To do this, the test sample is filled with sulfuric and nitric acid in a ratio of 1:1 (1 ml of sulfur and 1 ml of nitric acid), carefully heated until complete oxidation (Brown nitrogen oxides are released) without bringing to a boil.
3.the entire mixture inside the bottle is diluted with distilled water and poured into a measuring bottle of 25 ml. It is necessary to filter the resulting solution when pouring it into a measuring vial, since some insoluble silicates remain after the oxidation process.
4.to determine the amount of lead by absorption method, it is necessary to pass the vial to a laboratory assistant.
5.drawing a gauge graph according to the resulting diagram, calculating the amount of lead in the studied solution. Calculation of the amount of lead per unit mass of dried dry plant material.
Control questions for the transfer of work:
drawing a gauge graph, calculating the lead value, comparing the obtained result with the results of other students in the group.
Give written answers to the following questions:
a) Where does lead come from on the leaves of plants in your opinion?
ə) does the amount of lead in plants depend on the area sampled?
B) state possible ways in which the lead in the leaves migrates.
Note: if the lead content in the solution is lower than the sensitivity of the instrument, the solution is evaporated 2-4 times and the measurement is repeated.
The order of work in the auditorium
In the chemistry laboratory, students must necessarily appear in white coats;
The student must come to class ready-made by writing laboratory work in a notebook or A4 sheet;
Students are offered to study the basic concepts of laboratory work on additional materials;
After reading the necessary information, students will be able to work in the laboratory with the necessary equipment and reagents;
Laboratory work must be done by each student and the result of laboratory work is recorded in a notebook or on A4
Laboratory work №9
Determination of environmental pollutants
Lesson plan:
1. effect of heavy metal ions on aquatic organisms
2. cleaning the Cu2+ ion contained in wastewater with natural sorbents
Purpose of the work.
1.familiarization with the principles of the Biotesting method.
2.perform a simple static experiment on objects that are easy to find.
3. the value of LS50 for interval time 2 and 24 hours __________calculation.
Chemical vessels and equipment:
1. Petri dishes or chemical glass
2. scales
3. centrifuge
Reagents.
1.CuSO4
2.H2O
3.0, 5 g of grated natural sorbent per kilogram
4. talc
5.0. 5 g of ground chalk
6. pH paper
Progress of work
1. preparation of heavy metal salt solutions (according to the teacher). Concentrations of solutions calculated by metal cation 1; 2; 3; 4; 8; 10 let it be mg / l.
2.Prepare 6 Petri dishes (you can also use chemical glasses) thoroughly washed. Five of the containers are filled with prepared solutions, and the sixth is filled with distilled water.
In each vessel, it is necessary to put two of the living organisms intended for study of the same dimensions. After half an hour of observation, the replacement of a living organism that has noticed an unpleasant change.
3.record the death of each vessel at a certain time interval (15 minutes; 0.5; 1; 2; 24 hours). In the ideal variant, one concentration value must be determined. When the concentration is higher than this value, there must be 100% mortality, and when it is lower, there must be no mortality. Recording the received data in a table.
Warning. There must be no death in the container for comparison. In this case, it is necessary to repeat the experiment. If the concentration value mentioned above is not found, it is necessary to repeat the experiment. In repeated experiments, the concentration value is increased by 5-20 times.
4.graph representation of the concentration dependence of the proportion of species in which death occurred at intervals of 2 and 24 hours of time. Determination of the LC50 metallic concentration value on the graph for 50% of species.
To hand over the work.
1. table of experimental results;
2. graphical interpretation of data;
3.lc50 value for two time intervals.
4. give written answers to control questions:
a) what is LC50 and LD50?
ə) comparison of the limit quantitative concentration (CMC) of the metal used in the experiment with the value obtained by you LC50.
B) What is" Threshold"," lethal concentration"?
Is it possible to determine it as a result of your experiment?
B) cleaning the Cu2+ ion contained in wastewater with natural sorbents
The composition of wastewater can be found in the wastewater of copper mining chemical industries, artificial fiber factory wastewater, wastewater of agricultural and other industries. The toxicity of concentrated (1 mg/L 5 g/l) copper depends on the state in which the metal is found, depending on the type of chemical background. A change in the state of the metal has a great effect on its toxicity. Fe2O3 and metal coated with humic acid adsorbed on natural sorbent particles reduce or eliminate the toxicity property, as the high adsorption ion exchange filtration property and low cost allow the natural dispersed mineral to be widely used in Environmental Protection. Depending on the composition of natural sorbents:
1. dispersed silica (amorphous silica);
2. layered tape and layered minerals (talc);
3. structural silicates are divided into three large groups.
The concentration of CU2 + ion CMC is 1 mg / l.
Progress of work.
1) Take 3-50 and 100 ml glasses and pour CuSO4 • 5 H2O each.
2) for 1 glass – weigh on a scale and add a natural sorbent, grated in 0.5 g per kilogram.
3) add the same amount of talcum powder to the glass.
4) add 0.5 g of ground chalk to the glass.
5) stir intensively for 1 minute. Pour the resulting solution and separate it from the precipitate in a centrifuge.
6) filter the solution in a centrifuge in a separate vial of 25-50 ML.
7) Universal pH paper and determine the pH of each solution.
8) mark solutions with changed ph. What is it connected with?
Fill in the resulting result in the table
|
№ |
Sorbents |
рН |
|
1. |
|
|
|
2. |
|
|
|
3. |
|
|
Laboratory work №10
Determination of the total sulfur content of coal
Lesson plan:
1. determination of the amount of sulfur
2. determination of ash content (ash content) of coal
Purpose of the work. Determination of the total sulfur content of coal
Reagents and equipment:
1.1 g Eshik mixture (60% MgO 40% Na2CO3)
2. Crucible
3. muffle oven
4. analytical scales
5.10%-vertical barium chloride solution
Progress of work
1.about 1 g of coal samples are measured with an accuracy of 0.0001 G, 1 g of Ashik mixture (60% MgO 40% Na2CO3) is measured with an accuracy of 0.1 g, placed in a crucible and mixed with a glass rod. Then take another 1 g of Ashik mixture and sprinkle it on top of the mixture in the Crucible.
2.after the Crucible muffle is heated for 2 hours in the oven at 850+- 250oc, it is heated for another 2-2. 5 hours at this temperature.After cooling, the mixture in the Crucible is loosened with a stick, placed in a 300 ml glass, 100-150 ml of hot water is added to it and heated to a boil so that no lumps of the mixture remain on the walls. If unburned coal pellets come out on the surface of the mixture,
there he repeats the analysis. If the Crucible is difficult to wash, then it is placed in the same glass and boiled together with the Crucible.
3.decontaminate the aqueous solution and transfer it to filter paper, collecting the precipitate (filtrate) in a 500 ml glass. The sediment remaining in the glass is rinsed with hot distilled water and transferred to a filter. In the solution (filtrate) above the resulting precipitate (in a volume of about 350 ml), add 2-3 drops of methylorange and hydrochloric acid until a weakly acidic medium is formed.
4.the solution is heated to a boil and 10 ml of a 10% solution of barium chloride heated to a boil is added to it, as a result of which BaSO4 ↓ keeps the precipitated solution in an aqueous heater close to the boiling point of 0.5-2. Then the solution in the glass is filtered with a dense filter paper and the sediment is washed off with hot water until a trace of chloride is removed from the filter. (checking the chlorine ion with a solution of silver nitrate).
5.the tincture is placed in a pre-measured Crucible, pressed tightly and heated in a spirit (pre-measured Crucible). The Crucible is heated in an 8500c muffle oven for 30-40 min.
Then the Crucible is removed from the muffle, cooled in the air for about 5 minutes, placed in the desiccator and the heating can be repeated. The heating is stopped when the mass loss is about 0.001 G.
6.measures the amount of sulfur in the measurement ( % ).
2. determination of ash content (ash content) of coal
Coal ashing is carried out in a ceramic Crucible with a diameter of d 25-35 mm and d=35-45 mm. It is advisable to take the Crucible brought by heating to a constant mass from the desiccator.
Progress of work
1-2 g of coal from the sample is weighed on an analytical scale with an accuracy of 0.0001 G and placed in a muffle furnace, pre-heated to 2000oc, and a constant temperature is set. The muffle is heated in the oven for 1-2 hours to 800-8300oc and again at this temperature for 1-2 hours. The Crucible is removed from the muffle by grasping it with tongs, held in the air for about 5 minutes, and then cooled to room temperature and placed in a desiccator. After a while, you can measure. When very accurate determinations need to be made, additional heats can be controlled by repeatedly measuring the Crucible with the ash every 30 minutes.
It is necessary to repeat these measurements until the decrease (change) in mass reaches 0.001 G.
The ash content can be determined (calculated ) by the
equation below.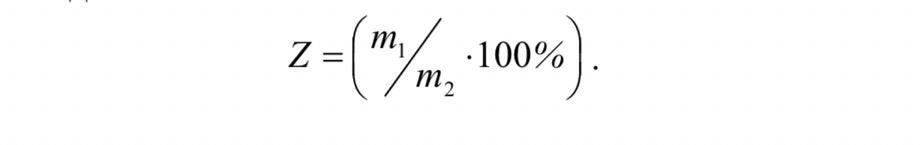 Where: m1 is the mass of ash, m2 is the mass of
coal.
Where: m1 is the mass of ash, m2 is the mass of
coal.
Laboratory work №11
Determination of sulfur dioxide and chlorine in the laboratory
Lesson plan:
1. determination of sulfur dioxide
2. determination of chlorine in the laboratory
Purpose of the work. Determination of sulfur dioxide and chlorine
Equipment:
1) absorbent containers (absorbents);
2) aspirator;
3) Selicon tubes;
Reagents:
1) iodine (0.0001% Iodine solution)
2) starch (0.3% solution).
Progress of work
Pour a mixture of 1 ml of 0.0001% Iodine solution and 1-2 drops of 0.3% starch solution into the absorbent. Filling distilled water into a graduated glass container with a Tubus at the bottom.
Pouring water from the tap of the Tubus at a speed of 10 mL/min. At this speed, counting bubbles passing through the absorber is not a problem. As a result of air thinning during the discharge of water, air is freely pumped out. The experiment is carried out until the color of the absorbent solution disappears. The volume of air that has passed through the absorber can be determined by the volume of water that has left the aspirator.
The concentration of sulfur dioxide in the air can be determined using Table 1.
-
Air volume
(ML)
Sulfur gas
concentration,
m mg / m3
Air
volume
(ML)
Sulfur gas
volume,
mg / m3
10
320
100
32
20
160
110
29
30
107
120
27
40
80
130
24
50
64
140
22
60
53
150
20
70
46
200
16
80
40
250
12
90
35
300
10
The principle of the method.
It is based on the reduction of iodine by sulfur gas to iodine hydrogen.
J2+ H2O + SO2= SO3 + 2HJ.
Determination of chlorine in the laboratory
Equipment:
1) indicator Tube, 2) aspirator.
a) reagents, No. 1-solution reagent, 2) indicator powder.
Preparation of reagent No. 1. 30 g of potassium bromide dissolve 1 g of potassium carbonate in 100 ml of distilled water and add 0.1 g of fluorescein (previously dissolved in 1 ml of 10% potassium hydroxide).
powder indicator: 1 g of Slicogel is treated with 1.2 ml of reagent No. 1 and dried in a drying cabinet for 30 minutes. Indicator tube No. 1-slicogel, in which the reagent is absorbed, begins to turn red in accordance with the chlorine concentration as a result of the absorption of chlorine in the air. The sensitivity of the approach is 0.002 mg/l. bromides are hindered by substances that displace bromine.
Determination process: through the indicator tube into which the prepared powder is placed, 300 cm3 of air is pumped out using an aspirator. The observation of a red color indicates the presence of a chlorine ion in the air.
2) determination of the indicator using paper
Equipment: filter paper strips.
Reagents; No. 2-reagent solution.
Preparation of reagent No. 2. 30 g of potassium bromide (KBr), 2 g of potassium carbonate (K2CO3) are prepared by adding 0.2 g of fluorescein solution to a solution of 10 ml of glycerin in 250 ml of water (0.2 g of fluorescein is dissolved in 2 ml of 10% Con or Naoh solution). Drying in the fresh air by soaking in filter strips from the resulting solution. When fluorescein potassium bromide-based filter paper comes into contact with chlorine, it turns yellow. When the chlorine concentration is 0.03 mg/l, The Purple color becomes noticeable after a few seconds.
3) determination of iodine by starch paper
Equipment: filter paper strips .
Reagents; 0.2% starch solution 0.5 potassium iodide solution
Preparation of the indicator solution. Filter paper strips. It is impregnated with a 5% solution of potassium iodide and 0.2% starch and dried in the air. The colors should be white. The reaction is observed in the manifestation of the blue color of chlorine displacing iodine in potassium iodide as a result of the interaction of the released iodine with starch (or black color). The color is noticeable in 10 minutes when the chlorine concentration is 0.0014 mg/l. Bromine oxide and other oxidants lead to detection.
Laboratory work №12
Determination of sulfur in drinks
Lesson plan:
1. Environmental Chemistry of foods.
2. sulfur dioxide
3. determination of ammonia nitrogen in meat products
Purpose of the work. Determination of sulfur in beverages, artificial dyes and ammonia nitrogen in various poisons.
Reagents and equipment:
1. conical bottle
2.100 ML degassing drink
3. 5 ml 2m NaOH solution
4. starch 1 ml solution
5. 0 ML 2m H2SO4 solution
6. 0.001 Iodine solution
7. sulfur dioxide concentration
Brief theoretical information.
Environmental chemistry of foods. In modern times, various chemicals are added to foods to preserve them for a long time, preservatives, antioxidants, dyes, emulsifiers are added to give food a sweet or sour taste, vitaminize and affect the smell of food.
Coloring substances improve the appearance of dishes. While a number of Coloring substances are natural, most are artificial, they are obtained from coal and oil products. For example, when comparing data on red coloring substances obtained over the past thirty years, interesting facts were revealed. Previously widely used dyes are now considered carcinogenic.
Let's take the substance "Orange B" for a typical example as an artificial coloring agent. This is due to the presence of aromatic nuclei in the composition of the dye of the substance. On the other hand, the fusion of these nuclei and the-N-N - fragment is similar to the structure of some carcinogenic substances.
The principle of the method.
The amount of sulfur dioxide is determined by the reaction
of interaction with iodine: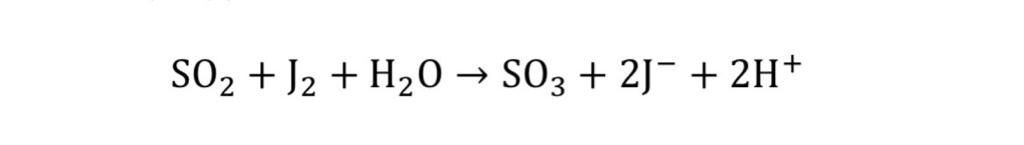 Progress of work
Progress of work
1.pour 100 ml of the carbonated drink into a conical vial, add 5 ml of 2m NaOH solution and leave for 30 minutes.
2.Add 1 ml of freshly prepared starch solution, 10 ml of 2m H2SO4 solution to the vial and immediately titrate with 0.001 Iodine solution until a non-fading blue color appears.
3.calculation of the concentration of sulfur dioxide in milligrams per 1 L of the drink.
To pass the work, you need to compare the results of the calculation with the drinks of other students in the group:
In what foods are artificial dyes used? - preparation of a written answer to the question.
Determination of ammonia nitrogen in meat products
Determination of ammonia nitrogen in meat products by titration with phenolphthalein.
Progress of work
The crushed meat is taken 20 g in a 150-200 ML-liter cone-shaped bottle, 80 ml of distilled water is added to it and filtered, shaking for 3-5 minutes. Take 10 ml of filtered water, add 40 ml of distilled water, 2-3 drops of 1% phenolphthalein and 10 ml of 40% formalin solution and titrate with 0.1 n sodium hydroxide to pink color.
The amount of ammonia nitrogen in 10 ml of water is determined by multiplying by a factor of 1.4 and 0.1 N by the volume of sodium hydroxide spent on titration. The content of ammonia nitrogen in fresh meat will be about 1.26 mg, in old meat 1.27-1.65, in very old meat 1.70 mg.
The order of work in the auditorium
In the chemistry laboratory, students must necessarily appear in white coats;
The student must come to class ready-made by writing laboratory work in a notebook or A4 sheet;
Students are offered to study the basic concepts of laboratory work on additional materials;
After reading the necessary information, students will be able to work in the laboratory with the necessary equipment and reagents;
Laboratory work must be done by each student and the result of laboratory work is recorded in a notebook or on A4 sheet.
Procedure for creating reports and protecting them
The report is drawn up in the following order:
1. protection of laboratory work and compliance with safety rules when performing work
2. use of reagents with the necessary equipment
3. pay attention to the crystallization of the salt and see if it is cleaned
4. registration and submission of work on A4 sheet
Laboratory work №13
Analysis of the composition of some food products. Determination of iron (GE) in vegetables
Lesson plan:
1.analysis of the composition of some food products.
2. determination of Iron (GE) in vegetables
Purpose of the work. Determination of iron (GE) in vegetables
Chemical vessels and equipment:
1. muffle oven
2. Crucible
3. Test
Reagents.
5 ml of water (distilled)
5 ml 0.1 m NH4CNS solution per filtrate
10 ml 2m HCl solution
Gecl3 6 color standard solution
GE3+(1m) 1 L solution
0.1 m 5 ml NH4CNS solution
leaf stalks
Progress of work
1.Gecl3 6 color standard solution preparation. GE3+(1m) preparation of 1 L of the solution, by dilution from 100 ml 0,01%, 0,005; 0,0025; 0,0005; 0,00025; 0,000125 %; 0.
2.Pour 0.1 m 5 ml of NH4CNS solution into the test tube from each solution.
3.keep the sample particles crushed.
4.put 2.5 G in individual crucibles (leaf stalks).
5.heat the samples in the muffle oven until dark blue.
6.after cooling the contents of the Crucible, place in a 50 ml glass and add 10 ml of 2m HCl solution to 5 ml of water (distilled) with intensive stirring for 1 minute.
7.collect a means for filtering. Place a tester under the caster.
8.pour the filtrate from the glass into the filter and take 5 ml of filtrate into the test tube.
9.add 5 ml of 0.1 m NH4CNS solution to the filtrate, Cork and stir vigorously with a whisk.
10.compare the resulting solution with the standard one.
11.describe the concentration of the GE ion in the test solution.
12.check the result obtained by measuring it on a spectrophotometer (l=490 Nm).
13.comparison of the result obtained with the results of students in the course.
Laboratory work №14
Determination of pollutant components in the atmosphere
Determination of air dustiness
Lesson plan:
1. determination of pollutant components in the atmosphere
2. determination of air dustiness
Purpose of the work. Air dustiness determination by comparing the leaves of trees growing along the road and away from the road
Reagents and equipment:
1. fine portioned filter
2. pump
3. glass plates measuring 5x7. 5 cm
4. microscope
5. filter
5. Leaf
Progress of work
1.installation of a fine-portioned filter in the inlet hole of the sampling pump (probe).
2.filter the air in the laboratory for 15 minutes.
3.in the same way, 15 minutes of suction from the air in the field.
4.Place the filters on a glass surface 5x7. 5 cm in size and look under a microscope. Count the fractions in each square.
5.determining the average number of particles in each square, multiplying it by the number of squares, is determined by the number of particles in the area of 2 cm2, that is, on the filter surface.
6. identify
a) how many particles are in the area of 9 cm2;
ə) how many particles are in 1cm3 of air;
B) determine the particle diameter of any particle at least 20;
7. calculate the percentage
a) large dust particles (10 microns);
ə) fine dust particles (1-10 microns);
B)aerosols (1 micron);
Laboratory work №15
Oil products in the soil (aromatic hydrocarbons)
detection method
Lesson plan:
1. gasoline
2. benzene
Purpose of the work. Gasochromatographic determination of the vapor phase of equilibrium in a flame ionizing detector, concentrating gasoline in the soil
Reagents and equipment:
- Flame-ionizing detector chromatograph chromatographic column made of stainless steel with a length of 2 m with a diameter of 3 mm;
- dryer cabinet or ultramicrothermostat;
-45 ml glass containers;
- micro print;
- medical syringe 5 ml;
- gasoline a-80;
- ethanol 96%;
- acetone;
- nitrogen, air, hydrogen gases (in gear cylinders).
Standard gasoline solutions in the amount of 0.01 mg/mL.
1 mg of gasoline is dissolved in etonol in a 100 ml bottle. Working standard gasoline solutions 0,05; 0,025; 0,5; 0,75; 1,0; 1,25; 1,50 mcg / ml, prepared by diluting the original standard gasoline solutions with water.
Distilled water. Inerton AW-DMCS. Trypropionitrylamine (TPNA). At the end connected to the chromatographic column, AW-DMCS consists of TPNA, which accounts for 20% of the inert mass. TPNA is dissolved in acetone and filled with a solid medium. The mixture is thoroughly mixed until the solvent is completely gone.
The chromatographic column is filled with dry absorbent, covered with glass cotton at both ends and concentrated at 100oc for 10 hours without connecting to the chromatograph thermostat to the detector.
Gradation graph.
In glass containers, 10 grams of soil are placed for control, 2 ml of standard working solutions are added, which are used for the production of gasoline 0; 0,1; 0,5; 1,0; 1,5; 2,0; 2,5; 3,0 corresponds to MCG. The dishes are shaken well, corked and left for 1 hour. Processes standards under test conditions. For chromatographic separation, 5 ml of vapor phase is poured into this emitter, the area of the peaks of the standards is calculated from the chromatogram and five
according to the average determination, a graph is drawn of the relationship between the area of the rods and the amount of gasoline (mm2).
Progress of work.
In a container brought to a constant weight, 10 g of soil is placed at the place of sampling and sent to the laboratory in a stopper. At the same time, the soil is taken to determine the moisture content of the soil. It is advisable to analyze the sample on the day it was taken.
Weighing the container with its soil, a sample is calculated based on the difference in masses. The container should be placed in a 100oc thermostat for 15 minutes and shaken 1-2 times during heating.
Based on the equilibrium concentration of benzene in the soil and chromatographic determination of the equilibrium vapor phase in a flame-ionizing detector device.
The lower detection threshold in the soil is 0.1 MCG , the analysis accuracy is ±8%, the measured concentrations are 0.01-1.0 mg/kg.
Acetone, isopropylbenzene, styrene, toluene do not interfere with determination.
Tools and reagents.
See the tools in the method above.
- Benzene, ethanol 96%, chloroform.
- Gases in the balloon-nitrogen, hydrogen, air.
- Standard benzene solutions containing 10 µg/mL of benzene.
Benzene is prepared by dissolving in ethanol in a 100 mL vial.
Benzene 0,05; 0,1; 0,15; 0,20; 0,25; 0,30; 0,35; 0,40; 0,45; 0,5 standard mcg / ml solutions are prepared by diluting the original standard solution with water.
Chromaton N-AW, 0.20-0.25 mm fraction.
Polyethylene glycol 20,000 (PEG).
At the end of the chromatograph column, polyethylene glycol 20,000 is absorbed into the chromaton n-AW and filled with 15% of the mass of the carrier. PEG is placed in a solution dissolved in chloroform with a solid media. The excess solvent is evaporated in the water heater, covering the entire solvent carrier and stirring until all the solvent evaporates. The filled column is sealed on both sides with a glass cloth, placed in the thermostat of the chromatograph, without connecting to the detector, the first 2 hours at 50 ° C, then 2 hours at 80 ° C and 7 hours at 120 ° C are concentrated in the carrier gas stream. In the working mode, connect the speaker to the detector and check the zero line.
Gradation graph.
In glass containers add 10 g from the Observer sample and 2 from the working standard solutions. He is a benzene 0,1; 0,2; 0,3; 0,4; 0,5; 0,6; 0,7; 0,8; 0,9; 0,10 corresponds to MCG.
The dishes are corked and left for 2 hours, stirring. Then it is analyzed with a thermostat under the conditions of the sample. From each vessel, 5 ml of the steam-air mixture is taken with a syringe and sent through the evaporator to the chromatograph column for analysis. Measuring the vertex area in the resulting chromatogram, a graph of the relationship between the vertex area (mm2) with the benzene content (MCG) is made from five average definitions.
Progress of work
It is necessary to analyze the sample on the day of receipt or no more than a day (storage in 2-3 OS). It is necessary to take 10 g of a finely dispersed soil sample, put it in a glass container, plug it into the thermostat for 10 minutes at 80 OS, and do not forget to stir it partially.
Without removing the container from the thermostat, under the same conditions, the steam-gas mixture is removed with a thermostated syringe through the rubber of the vessel stopper, washed 3-5 times with the steam-gas mixture and sent back to the container, then 5 ml of the gas-steam mixture is taken into the syringe and sent to the chromatograph evaporator. Before the analysis, the chromatograph is activated and prepared for working mode.
Temperature of thermostat speakers 80oc, evaporator 125oc, nitrogen consumption 20 mL/min, hydrogen – 25 ml/min, Air 200 mL/min; benzene retention time 3 min 50 sec.
In the resulting chromatogram, the areas of the peaks of the analyzed substances are measured. The amount of benzene in the sample is determined by the gradation graph.
жүктеу мүмкіндігіне ие боласыз
Бұл материал сайт қолданушысы жариялаған. Материалдың ішінде жазылған барлық ақпаратқа жауапкершілікті жариялаған қолданушы жауап береді. Ұстаз тілегі тек ақпаратты таратуға қолдау көрсетеді. Егер материал сіздің авторлық құқығыңызды бұзған болса немесе басқа да себептермен сайттан өшіру керек деп ойласаңыз осында жазыңыз
Химиялық экология тақырыбы, зертханалық жұмыс
Химиялық экология тақырыбы, зертханалық жұмыс
Laboratory work №8
Determination of lead in the leaves of trees growing in different areas of the city
Lesson plan:
1. determination of lead in the leaves of trees
Purpose of the work. Detection of lead in the leaves of trees growing in different areas of the city. Compare the results obtained, make predictions about the sources of pollution.
Chemical vessels and equipment:
Keldal vial
Reagents.
1 ml of sulfur and 1 ml of nitric acid
Lead
plant leaves
Brief theoretical information.
The principle of the method.
Determination of the amount of lead in plant leaves after acid treatment by atomic absorption spectrometry.
Progress of work
1.grind undigested plant material in a drying cabinet at 30-40oC and weigh 0.2 g from it.
2.The measured mass is decomposed by wet ashtray in a 300 ml Keldal vial. To do this, the test sample is filled with sulfuric and nitric acid in a ratio of 1:1 (1 ml of sulfur and 1 ml of nitric acid), carefully heated until complete oxidation (Brown nitrogen oxides are released) without bringing to a boil.
3.the entire mixture inside the bottle is diluted with distilled water and poured into a measuring bottle of 25 ml. It is necessary to filter the resulting solution when pouring it into a measuring vial, since some insoluble silicates remain after the oxidation process.
4.to determine the amount of lead by absorption method, it is necessary to pass the vial to a laboratory assistant.
5.drawing a gauge graph according to the resulting diagram, calculating the amount of lead in the studied solution. Calculation of the amount of lead per unit mass of dried dry plant material.
Control questions for the transfer of work:
drawing a gauge graph, calculating the lead value, comparing the obtained result with the results of other students in the group.
Give written answers to the following questions:
a) Where does lead come from on the leaves of plants in your opinion?
ə) does the amount of lead in plants depend on the area sampled?
B) state possible ways in which the lead in the leaves migrates.
Note: if the lead content in the solution is lower than the sensitivity of the instrument, the solution is evaporated 2-4 times and the measurement is repeated.
The order of work in the auditorium
In the chemistry laboratory, students must necessarily appear in white coats;
The student must come to class ready-made by writing laboratory work in a notebook or A4 sheet;
Students are offered to study the basic concepts of laboratory work on additional materials;
After reading the necessary information, students will be able to work in the laboratory with the necessary equipment and reagents;
Laboratory work must be done by each student and the result of laboratory work is recorded in a notebook or on A4
Laboratory work №9
Determination of environmental pollutants
Lesson plan:
1. effect of heavy metal ions on aquatic organisms
2. cleaning the Cu2+ ion contained in wastewater with natural sorbents
Purpose of the work.
1.familiarization with the principles of the Biotesting method.
2.perform a simple static experiment on objects that are easy to find.
3. the value of LS50 for interval time 2 and 24 hours __________calculation.
Chemical vessels and equipment:
1. Petri dishes or chemical glass
2. scales
3. centrifuge
Reagents.
1.CuSO4
2.H2O
3.0, 5 g of grated natural sorbent per kilogram
4. talc
5.0. 5 g of ground chalk
6. pH paper
Progress of work
1. preparation of heavy metal salt solutions (according to the teacher). Concentrations of solutions calculated by metal cation 1; 2; 3; 4; 8; 10 let it be mg / l.
2.Prepare 6 Petri dishes (you can also use chemical glasses) thoroughly washed. Five of the containers are filled with prepared solutions, and the sixth is filled with distilled water.
In each vessel, it is necessary to put two of the living organisms intended for study of the same dimensions. After half an hour of observation, the replacement of a living organism that has noticed an unpleasant change.
3.record the death of each vessel at a certain time interval (15 minutes; 0.5; 1; 2; 24 hours). In the ideal variant, one concentration value must be determined. When the concentration is higher than this value, there must be 100% mortality, and when it is lower, there must be no mortality. Recording the received data in a table.
Warning. There must be no death in the container for comparison. In this case, it is necessary to repeat the experiment. If the concentration value mentioned above is not found, it is necessary to repeat the experiment. In repeated experiments, the concentration value is increased by 5-20 times.
4.graph representation of the concentration dependence of the proportion of species in which death occurred at intervals of 2 and 24 hours of time. Determination of the LC50 metallic concentration value on the graph for 50% of species.
To hand over the work.
1. table of experimental results;
2. graphical interpretation of data;
3.lc50 value for two time intervals.
4. give written answers to control questions:
a) what is LC50 and LD50?
ə) comparison of the limit quantitative concentration (CMC) of the metal used in the experiment with the value obtained by you LC50.
B) What is" Threshold"," lethal concentration"?
Is it possible to determine it as a result of your experiment?
B) cleaning the Cu2+ ion contained in wastewater with natural sorbents
The composition of wastewater can be found in the wastewater of copper mining chemical industries, artificial fiber factory wastewater, wastewater of agricultural and other industries. The toxicity of concentrated (1 mg/L 5 g/l) copper depends on the state in which the metal is found, depending on the type of chemical background. A change in the state of the metal has a great effect on its toxicity. Fe2O3 and metal coated with humic acid adsorbed on natural sorbent particles reduce or eliminate the toxicity property, as the high adsorption ion exchange filtration property and low cost allow the natural dispersed mineral to be widely used in Environmental Protection. Depending on the composition of natural sorbents:
1. dispersed silica (amorphous silica);
2. layered tape and layered minerals (talc);
3. structural silicates are divided into three large groups.
The concentration of CU2 + ion CMC is 1 mg / l.
Progress of work.
1) Take 3-50 and 100 ml glasses and pour CuSO4 • 5 H2O each.
2) for 1 glass – weigh on a scale and add a natural sorbent, grated in 0.5 g per kilogram.
3) add the same amount of talcum powder to the glass.
4) add 0.5 g of ground chalk to the glass.
5) stir intensively for 1 minute. Pour the resulting solution and separate it from the precipitate in a centrifuge.
6) filter the solution in a centrifuge in a separate vial of 25-50 ML.
7) Universal pH paper and determine the pH of each solution.
8) mark solutions with changed ph. What is it connected with?
Fill in the resulting result in the table
|
№ |
Sorbents |
рН |
|
1. |
|
|
|
2. |
|
|
|
3. |
|
|
Laboratory work №10
Determination of the total sulfur content of coal
Lesson plan:
1. determination of the amount of sulfur
2. determination of ash content (ash content) of coal
Purpose of the work. Determination of the total sulfur content of coal
Reagents and equipment:
1.1 g Eshik mixture (60% MgO 40% Na2CO3)
2. Crucible
3. muffle oven
4. analytical scales
5.10%-vertical barium chloride solution
Progress of work
1.about 1 g of coal samples are measured with an accuracy of 0.0001 G, 1 g of Ashik mixture (60% MgO 40% Na2CO3) is measured with an accuracy of 0.1 g, placed in a crucible and mixed with a glass rod. Then take another 1 g of Ashik mixture and sprinkle it on top of the mixture in the Crucible.
2.after the Crucible muffle is heated for 2 hours in the oven at 850+- 250oc, it is heated for another 2-2. 5 hours at this temperature.After cooling, the mixture in the Crucible is loosened with a stick, placed in a 300 ml glass, 100-150 ml of hot water is added to it and heated to a boil so that no lumps of the mixture remain on the walls. If unburned coal pellets come out on the surface of the mixture,
there he repeats the analysis. If the Crucible is difficult to wash, then it is placed in the same glass and boiled together with the Crucible.
3.decontaminate the aqueous solution and transfer it to filter paper, collecting the precipitate (filtrate) in a 500 ml glass. The sediment remaining in the glass is rinsed with hot distilled water and transferred to a filter. In the solution (filtrate) above the resulting precipitate (in a volume of about 350 ml), add 2-3 drops of methylorange and hydrochloric acid until a weakly acidic medium is formed.
4.the solution is heated to a boil and 10 ml of a 10% solution of barium chloride heated to a boil is added to it, as a result of which BaSO4 ↓ keeps the precipitated solution in an aqueous heater close to the boiling point of 0.5-2. Then the solution in the glass is filtered with a dense filter paper and the sediment is washed off with hot water until a trace of chloride is removed from the filter. (checking the chlorine ion with a solution of silver nitrate).
5.the tincture is placed in a pre-measured Crucible, pressed tightly and heated in a spirit (pre-measured Crucible). The Crucible is heated in an 8500c muffle oven for 30-40 min.
Then the Crucible is removed from the muffle, cooled in the air for about 5 minutes, placed in the desiccator and the heating can be repeated. The heating is stopped when the mass loss is about 0.001 G.
6.measures the amount of sulfur in the measurement ( % ).
2. determination of ash content (ash content) of coal
Coal ashing is carried out in a ceramic Crucible with a diameter of d 25-35 mm and d=35-45 mm. It is advisable to take the Crucible brought by heating to a constant mass from the desiccator.
Progress of work
1-2 g of coal from the sample is weighed on an analytical scale with an accuracy of 0.0001 G and placed in a muffle furnace, pre-heated to 2000oc, and a constant temperature is set. The muffle is heated in the oven for 1-2 hours to 800-8300oc and again at this temperature for 1-2 hours. The Crucible is removed from the muffle by grasping it with tongs, held in the air for about 5 minutes, and then cooled to room temperature and placed in a desiccator. After a while, you can measure. When very accurate determinations need to be made, additional heats can be controlled by repeatedly measuring the Crucible with the ash every 30 minutes.
It is necessary to repeat these measurements until the decrease (change) in mass reaches 0.001 G.
The ash content can be determined (calculated ) by the
equation below.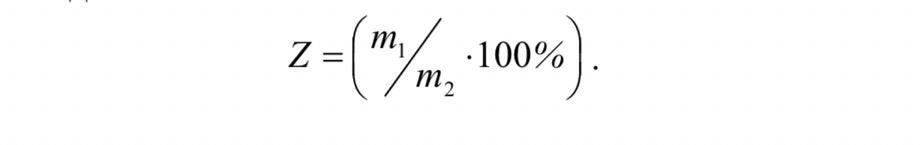 Where: m1 is the mass of ash, m2 is the mass of
coal.
Where: m1 is the mass of ash, m2 is the mass of
coal.
Laboratory work №11
Determination of sulfur dioxide and chlorine in the laboratory
Lesson plan:
1. determination of sulfur dioxide
2. determination of chlorine in the laboratory
Purpose of the work. Determination of sulfur dioxide and chlorine
Equipment:
1) absorbent containers (absorbents);
2) aspirator;
3) Selicon tubes;
Reagents:
1) iodine (0.0001% Iodine solution)
2) starch (0.3% solution).
Progress of work
Pour a mixture of 1 ml of 0.0001% Iodine solution and 1-2 drops of 0.3% starch solution into the absorbent. Filling distilled water into a graduated glass container with a Tubus at the bottom.
Pouring water from the tap of the Tubus at a speed of 10 mL/min. At this speed, counting bubbles passing through the absorber is not a problem. As a result of air thinning during the discharge of water, air is freely pumped out. The experiment is carried out until the color of the absorbent solution disappears. The volume of air that has passed through the absorber can be determined by the volume of water that has left the aspirator.
The concentration of sulfur dioxide in the air can be determined using Table 1.
-
Air volume
(ML)
Sulfur gas
concentration,
m mg / m3
Air
volume
(ML)
Sulfur gas
volume,
mg / m3
10
320
100
32
20
160
110
29
30
107
120
27
40
80
130
24
50
64
140
22
60
53
150
20
70
46
200
16
80
40
250
12
90
35
300
10
The principle of the method.
It is based on the reduction of iodine by sulfur gas to iodine hydrogen.
J2+ H2O + SO2= SO3 + 2HJ.
Determination of chlorine in the laboratory
Equipment:
1) indicator Tube, 2) aspirator.
a) reagents, No. 1-solution reagent, 2) indicator powder.
Preparation of reagent No. 1. 30 g of potassium bromide dissolve 1 g of potassium carbonate in 100 ml of distilled water and add 0.1 g of fluorescein (previously dissolved in 1 ml of 10% potassium hydroxide).
powder indicator: 1 g of Slicogel is treated with 1.2 ml of reagent No. 1 and dried in a drying cabinet for 30 minutes. Indicator tube No. 1-slicogel, in which the reagent is absorbed, begins to turn red in accordance with the chlorine concentration as a result of the absorption of chlorine in the air. The sensitivity of the approach is 0.002 mg/l. bromides are hindered by substances that displace bromine.
Determination process: through the indicator tube into which the prepared powder is placed, 300 cm3 of air is pumped out using an aspirator. The observation of a red color indicates the presence of a chlorine ion in the air.
2) determination of the indicator using paper
Equipment: filter paper strips.
Reagents; No. 2-reagent solution.
Preparation of reagent No. 2. 30 g of potassium bromide (KBr), 2 g of potassium carbonate (K2CO3) are prepared by adding 0.2 g of fluorescein solution to a solution of 10 ml of glycerin in 250 ml of water (0.2 g of fluorescein is dissolved in 2 ml of 10% Con or Naoh solution). Drying in the fresh air by soaking in filter strips from the resulting solution. When fluorescein potassium bromide-based filter paper comes into contact with chlorine, it turns yellow. When the chlorine concentration is 0.03 mg/l, The Purple color becomes noticeable after a few seconds.
3) determination of iodine by starch paper
Equipment: filter paper strips .
Reagents; 0.2% starch solution 0.5 potassium iodide solution
Preparation of the indicator solution. Filter paper strips. It is impregnated with a 5% solution of potassium iodide and 0.2% starch and dried in the air. The colors should be white. The reaction is observed in the manifestation of the blue color of chlorine displacing iodine in potassium iodide as a result of the interaction of the released iodine with starch (or black color). The color is noticeable in 10 minutes when the chlorine concentration is 0.0014 mg/l. Bromine oxide and other oxidants lead to detection.
Laboratory work №12
Determination of sulfur in drinks
Lesson plan:
1. Environmental Chemistry of foods.
2. sulfur dioxide
3. determination of ammonia nitrogen in meat products
Purpose of the work. Determination of sulfur in beverages, artificial dyes and ammonia nitrogen in various poisons.
Reagents and equipment:
1. conical bottle
2.100 ML degassing drink
3. 5 ml 2m NaOH solution
4. starch 1 ml solution
5. 0 ML 2m H2SO4 solution
6. 0.001 Iodine solution
7. sulfur dioxide concentration
Brief theoretical information.
Environmental chemistry of foods. In modern times, various chemicals are added to foods to preserve them for a long time, preservatives, antioxidants, dyes, emulsifiers are added to give food a sweet or sour taste, vitaminize and affect the smell of food.
Coloring substances improve the appearance of dishes. While a number of Coloring substances are natural, most are artificial, they are obtained from coal and oil products. For example, when comparing data on red coloring substances obtained over the past thirty years, interesting facts were revealed. Previously widely used dyes are now considered carcinogenic.
Let's take the substance "Orange B" for a typical example as an artificial coloring agent. This is due to the presence of aromatic nuclei in the composition of the dye of the substance. On the other hand, the fusion of these nuclei and the-N-N - fragment is similar to the structure of some carcinogenic substances.
The principle of the method.
The amount of sulfur dioxide is determined by the reaction
of interaction with iodine: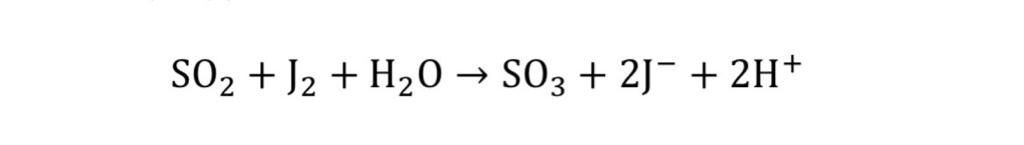 Progress of work
Progress of work
1.pour 100 ml of the carbonated drink into a conical vial, add 5 ml of 2m NaOH solution and leave for 30 minutes.
2.Add 1 ml of freshly prepared starch solution, 10 ml of 2m H2SO4 solution to the vial and immediately titrate with 0.001 Iodine solution until a non-fading blue color appears.
3.calculation of the concentration of sulfur dioxide in milligrams per 1 L of the drink.
To pass the work, you need to compare the results of the calculation with the drinks of other students in the group:
In what foods are artificial dyes used? - preparation of a written answer to the question.
Determination of ammonia nitrogen in meat products
Determination of ammonia nitrogen in meat products by titration with phenolphthalein.
Progress of work
The crushed meat is taken 20 g in a 150-200 ML-liter cone-shaped bottle, 80 ml of distilled water is added to it and filtered, shaking for 3-5 minutes. Take 10 ml of filtered water, add 40 ml of distilled water, 2-3 drops of 1% phenolphthalein and 10 ml of 40% formalin solution and titrate with 0.1 n sodium hydroxide to pink color.
The amount of ammonia nitrogen in 10 ml of water is determined by multiplying by a factor of 1.4 and 0.1 N by the volume of sodium hydroxide spent on titration. The content of ammonia nitrogen in fresh meat will be about 1.26 mg, in old meat 1.27-1.65, in very old meat 1.70 mg.
The order of work in the auditorium
In the chemistry laboratory, students must necessarily appear in white coats;
The student must come to class ready-made by writing laboratory work in a notebook or A4 sheet;
Students are offered to study the basic concepts of laboratory work on additional materials;
After reading the necessary information, students will be able to work in the laboratory with the necessary equipment and reagents;
Laboratory work must be done by each student and the result of laboratory work is recorded in a notebook or on A4 sheet.
Procedure for creating reports and protecting them
The report is drawn up in the following order:
1. protection of laboratory work and compliance with safety rules when performing work
2. use of reagents with the necessary equipment
3. pay attention to the crystallization of the salt and see if it is cleaned
4. registration and submission of work on A4 sheet
Laboratory work №13
Analysis of the composition of some food products. Determination of iron (GE) in vegetables
Lesson plan:
1.analysis of the composition of some food products.
2. determination of Iron (GE) in vegetables
Purpose of the work. Determination of iron (GE) in vegetables
Chemical vessels and equipment:
1. muffle oven
2. Crucible
3. Test
Reagents.
5 ml of water (distilled)
5 ml 0.1 m NH4CNS solution per filtrate
10 ml 2m HCl solution
Gecl3 6 color standard solution
GE3+(1m) 1 L solution
0.1 m 5 ml NH4CNS solution
leaf stalks
Progress of work
1.Gecl3 6 color standard solution preparation. GE3+(1m) preparation of 1 L of the solution, by dilution from 100 ml 0,01%, 0,005; 0,0025; 0,0005; 0,00025; 0,000125 %; 0.
2.Pour 0.1 m 5 ml of NH4CNS solution into the test tube from each solution.
3.keep the sample particles crushed.
4.put 2.5 G in individual crucibles (leaf stalks).
5.heat the samples in the muffle oven until dark blue.
6.after cooling the contents of the Crucible, place in a 50 ml glass and add 10 ml of 2m HCl solution to 5 ml of water (distilled) with intensive stirring for 1 minute.
7.collect a means for filtering. Place a tester under the caster.
8.pour the filtrate from the glass into the filter and take 5 ml of filtrate into the test tube.
9.add 5 ml of 0.1 m NH4CNS solution to the filtrate, Cork and stir vigorously with a whisk.
10.compare the resulting solution with the standard one.
11.describe the concentration of the GE ion in the test solution.
12.check the result obtained by measuring it on a spectrophotometer (l=490 Nm).
13.comparison of the result obtained with the results of students in the course.
Laboratory work №14
Determination of pollutant components in the atmosphere
Determination of air dustiness
Lesson plan:
1. determination of pollutant components in the atmosphere
2. determination of air dustiness
Purpose of the work. Air dustiness determination by comparing the leaves of trees growing along the road and away from the road
Reagents and equipment:
1. fine portioned filter
2. pump
3. glass plates measuring 5x7. 5 cm
4. microscope
5. filter
5. Leaf
Progress of work
1.installation of a fine-portioned filter in the inlet hole of the sampling pump (probe).
2.filter the air in the laboratory for 15 minutes.
3.in the same way, 15 minutes of suction from the air in the field.
4.Place the filters on a glass surface 5x7. 5 cm in size and look under a microscope. Count the fractions in each square.
5.determining the average number of particles in each square, multiplying it by the number of squares, is determined by the number of particles in the area of 2 cm2, that is, on the filter surface.
6. identify
a) how many particles are in the area of 9 cm2;
ə) how many particles are in 1cm3 of air;
B) determine the particle diameter of any particle at least 20;
7. calculate the percentage
a) large dust particles (10 microns);
ə) fine dust particles (1-10 microns);
B)aerosols (1 micron);
Laboratory work №15
Oil products in the soil (aromatic hydrocarbons)
detection method
Lesson plan:
1. gasoline
2. benzene
Purpose of the work. Gasochromatographic determination of the vapor phase of equilibrium in a flame ionizing detector, concentrating gasoline in the soil
Reagents and equipment:
- Flame-ionizing detector chromatograph chromatographic column made of stainless steel with a length of 2 m with a diameter of 3 mm;
- dryer cabinet or ultramicrothermostat;
-45 ml glass containers;
- micro print;
- medical syringe 5 ml;
- gasoline a-80;
- ethanol 96%;
- acetone;
- nitrogen, air, hydrogen gases (in gear cylinders).
Standard gasoline solutions in the amount of 0.01 mg/mL.
1 mg of gasoline is dissolved in etonol in a 100 ml bottle. Working standard gasoline solutions 0,05; 0,025; 0,5; 0,75; 1,0; 1,25; 1,50 mcg / ml, prepared by diluting the original standard gasoline solutions with water.
Distilled water. Inerton AW-DMCS. Trypropionitrylamine (TPNA). At the end connected to the chromatographic column, AW-DMCS consists of TPNA, which accounts for 20% of the inert mass. TPNA is dissolved in acetone and filled with a solid medium. The mixture is thoroughly mixed until the solvent is completely gone.
The chromatographic column is filled with dry absorbent, covered with glass cotton at both ends and concentrated at 100oc for 10 hours without connecting to the chromatograph thermostat to the detector.
Gradation graph.
In glass containers, 10 grams of soil are placed for control, 2 ml of standard working solutions are added, which are used for the production of gasoline 0; 0,1; 0,5; 1,0; 1,5; 2,0; 2,5; 3,0 corresponds to MCG. The dishes are shaken well, corked and left for 1 hour. Processes standards under test conditions. For chromatographic separation, 5 ml of vapor phase is poured into this emitter, the area of the peaks of the standards is calculated from the chromatogram and five
according to the average determination, a graph is drawn of the relationship between the area of the rods and the amount of gasoline (mm2).
Progress of work.
In a container brought to a constant weight, 10 g of soil is placed at the place of sampling and sent to the laboratory in a stopper. At the same time, the soil is taken to determine the moisture content of the soil. It is advisable to analyze the sample on the day it was taken.
Weighing the container with its soil, a sample is calculated based on the difference in masses. The container should be placed in a 100oc thermostat for 15 minutes and shaken 1-2 times during heating.
Based on the equilibrium concentration of benzene in the soil and chromatographic determination of the equilibrium vapor phase in a flame-ionizing detector device.
The lower detection threshold in the soil is 0.1 MCG , the analysis accuracy is ±8%, the measured concentrations are 0.01-1.0 mg/kg.
Acetone, isopropylbenzene, styrene, toluene do not interfere with determination.
Tools and reagents.
See the tools in the method above.
- Benzene, ethanol 96%, chloroform.
- Gases in the balloon-nitrogen, hydrogen, air.
- Standard benzene solutions containing 10 µg/mL of benzene.
Benzene is prepared by dissolving in ethanol in a 100 mL vial.
Benzene 0,05; 0,1; 0,15; 0,20; 0,25; 0,30; 0,35; 0,40; 0,45; 0,5 standard mcg / ml solutions are prepared by diluting the original standard solution with water.
Chromaton N-AW, 0.20-0.25 mm fraction.
Polyethylene glycol 20,000 (PEG).
At the end of the chromatograph column, polyethylene glycol 20,000 is absorbed into the chromaton n-AW and filled with 15% of the mass of the carrier. PEG is placed in a solution dissolved in chloroform with a solid media. The excess solvent is evaporated in the water heater, covering the entire solvent carrier and stirring until all the solvent evaporates. The filled column is sealed on both sides with a glass cloth, placed in the thermostat of the chromatograph, without connecting to the detector, the first 2 hours at 50 ° C, then 2 hours at 80 ° C and 7 hours at 120 ° C are concentrated in the carrier gas stream. In the working mode, connect the speaker to the detector and check the zero line.
Gradation graph.
In glass containers add 10 g from the Observer sample and 2 from the working standard solutions. He is a benzene 0,1; 0,2; 0,3; 0,4; 0,5; 0,6; 0,7; 0,8; 0,9; 0,10 corresponds to MCG.
The dishes are corked and left for 2 hours, stirring. Then it is analyzed with a thermostat under the conditions of the sample. From each vessel, 5 ml of the steam-air mixture is taken with a syringe and sent through the evaporator to the chromatograph column for analysis. Measuring the vertex area in the resulting chromatogram, a graph of the relationship between the vertex area (mm2) with the benzene content (MCG) is made from five average definitions.
Progress of work
It is necessary to analyze the sample on the day of receipt or no more than a day (storage in 2-3 OS). It is necessary to take 10 g of a finely dispersed soil sample, put it in a glass container, plug it into the thermostat for 10 minutes at 80 OS, and do not forget to stir it partially.
Without removing the container from the thermostat, under the same conditions, the steam-gas mixture is removed with a thermostated syringe through the rubber of the vessel stopper, washed 3-5 times with the steam-gas mixture and sent back to the container, then 5 ml of the gas-steam mixture is taken into the syringe and sent to the chromatograph evaporator. Before the analysis, the chromatograph is activated and prepared for working mode.
Temperature of thermostat speakers 80oc, evaporator 125oc, nitrogen consumption 20 mL/min, hydrogen – 25 ml/min, Air 200 mL/min; benzene retention time 3 min 50 sec.
In the resulting chromatogram, the areas of the peaks of the analyzed substances are measured. The amount of benzene in the sample is determined by the gradation graph.

шағым қалдыра аласыз