7.1.2.11 -элементтердің атауларын, валенттілікті және олардың қосылыстардағы атомдық қатынастарын қолдана отырып, биэлементті хими
7.1.2.11 -элементтердің атауларын, валенттілікті және олардың қосылыстардағы атомдық қатынастарын қолдана отырып, биэлементті хими


#1 слайд
8 grade
1 слайд
8 grade

#2 слайд
Сабақ тақырыбы
The theme of the lesson:
Иондық байланыс және коваленттік байланыс
Ionic bond and covalent bond
2 слайд
Сабақ тақырыбы The theme of the lesson: Иондық байланыс және коваленттік байланыс Ionic bond and covalent bond

#3 слайд
Сабақтың мақсаты
Lesson objectives:
Provide detailed information about the types
of chemical bonds, teach English terms
related to this topic
Химиялық байланыстың түрлері туралы толық ақпарат
беру, ағылшынша тақырыпқа байланысты терминдерді
үйрету
3 слайд
Сабақтың мақсаты Lesson objectives: Provide detailed information about the types of chemical bonds, teach English terms related to this topic Химиялық байланыстың түрлері туралы толық ақпарат беру, ағылшынша тақырыпқа байланысты терминдерді үйрету

#4 слайд
1. What particles do atoms work with to form a compound?
A) proton B) neutron C) electron d)nucleon
2. Who calculated the numerical value of electronegativity?
A) Lavoisier B)Pauling C)Priestley D)Mendeleev
3 . Specify the element with the highest electronegativity?
A) CL B) N C) F E) O
4. specify the element with the lowest electronegativity?
A) Cs B) Na C) K E) Ca
5. How does the electronegativity values of elements change over the period?
A) Decreases B)decreases, then increases C)does not change E) increases
6. How does the value of the electronegativity of elements change in the main
subgroups?
A) decrease B) increases, then decrease C) does not change E)
increases Алдыңғы білімді тексеру:
Activating previous knowledge:
« TEST »
https://wordwall.net/play/11533/562/903
4 слайд
1. What particles do atoms work with to form a compound? A) proton B) neutron C) electron d)nucleon 2. Who calculated the numerical value of electronegativity? A) Lavoisier B)Pauling C)Priestley D)Mendeleev 3 . Specify the element with the highest electronegativity? A) CL B) N C) F E) O 4. specify the element with the lowest electronegativity? A) Cs B) Na C) K E) Ca 5. How does the electronegativity values of elements change over the period? A) Decreases B)decreases, then increases C)does not change E) increases 6. How does the value of the electronegativity of elements change in the main subgroups? A) decrease B) increases, then decrease C) does not change E) increases Алдыңғы білімді тексеру: Activating previous knowledge: « TEST » https://wordwall.net/play/11533/562/903
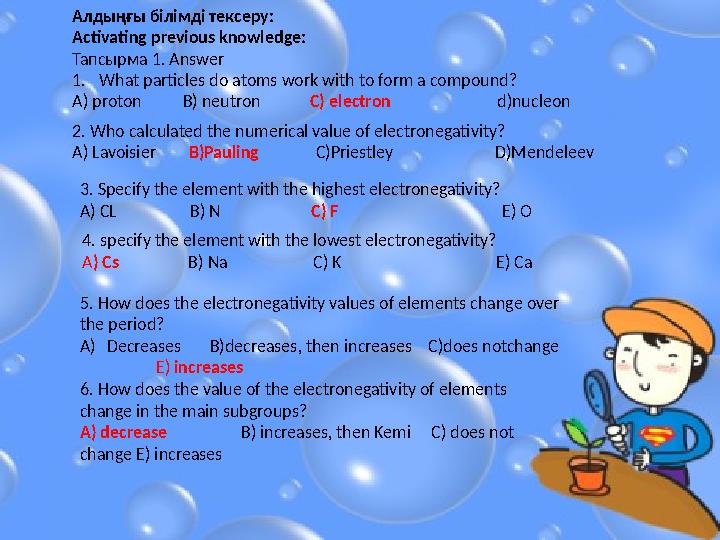
#5 слайд
1. What particles do atoms work with to form a compound?
A) proton B) neutron C) electron d)nucleonАлдыңғы білімді тексеру:
Activating previous knowledge:
Тапсырма 1. А nswer
2. Who calculated the numerical value of electronegativity?
A) Lavoisier B)Pauling C)Priestley D)Mendeleev
3. Specify the element with the highest electronegativity?
A) CL B) N C) F E) O
4. specify the element with the lowest electronegativity?
A ) Cs B) Na C) K E) Ca
5. How does the electronegativity values of elements change over
the period?
A) Decreases B)decreases, then increases C)does notchange
E) increases
6. How does the value of the electronegativity of elements
change in the main subgroups?
A) decrease B) increases, then Kemi C) does not
change E) increases
5 слайд
1. What particles do atoms work with to form a compound? A) proton B) neutron C) electron d)nucleonАлдыңғы білімді тексеру: Activating previous knowledge: Тапсырма 1. А nswer 2. Who calculated the numerical value of electronegativity? A) Lavoisier B)Pauling C)Priestley D)Mendeleev 3. Specify the element with the highest electronegativity? A) CL B) N C) F E) O 4. specify the element with the lowest electronegativity? A ) Cs B) Na C) K E) Ca 5. How does the electronegativity values of elements change over the period? A) Decreases B)decreases, then increases C)does notchange E) increases 6. How does the value of the electronegativity of elements change in the main subgroups? A) decrease B) increases, then Kemi C) does not change E) increases

#6 слайд
«Ionic Bond - #aumsum #kids #education #science
#learn» Show video
6 слайд
«Ionic Bond - #aumsum #kids #education #science #learn» Show video

#7 слайд
Fill in the blanks
Sodium atomic number 11 electronic
configuration 2.8.1. Sodium has an extra
electron in its outermost shell .
Chlorine atomic number 17 electronic
configuration 2.8.7. Chlorine has 7 electrons
in its outermost shell . Chlorine needs
1 electron to complete its outermost shell.
When sodium reacts with chlorine, sodium
donates electron to chlorine . Sodium becomes
positively charged ion ( Na+ )
Chlorine accepts an electron from sodium
Cl becomes negatively charged ion ( Cl- )
7 слайд
Fill in the blanks Sodium atomic number 11 electronic configuration 2.8.1. Sodium has an extra electron in its outermost shell . Chlorine atomic number 17 electronic configuration 2.8.7. Chlorine has 7 electrons in its outermost shell . Chlorine needs 1 electron to complete its outermost shell. When sodium reacts with chlorine, sodium donates electron to chlorine . Sodium becomes positively charged ion ( Na+ ) Chlorine accepts an electron from sodium Cl becomes negatively charged ion ( Cl- )

#8 слайд
Terminologi
Тұрақты электрон
stable electron
қосылыстар Compounds
Иондық
Ionic
Ковалентті
Covalent
Полюсті
polar
Полюссіз
nonpolar
Береді
give
Қосып алады
take
8 слайд
Terminologi Тұрақты электрон stable electron қосылыстар Compounds Иондық Ionic Ковалентті Covalent Полюсті polar Полюссіз nonpolar Береді give Қосып алады take

#9 слайд
Terminologi
https://wordwall.net/play/11542/782/413
9 слайд
Terminologi https://wordwall.net/play/11542/782/413
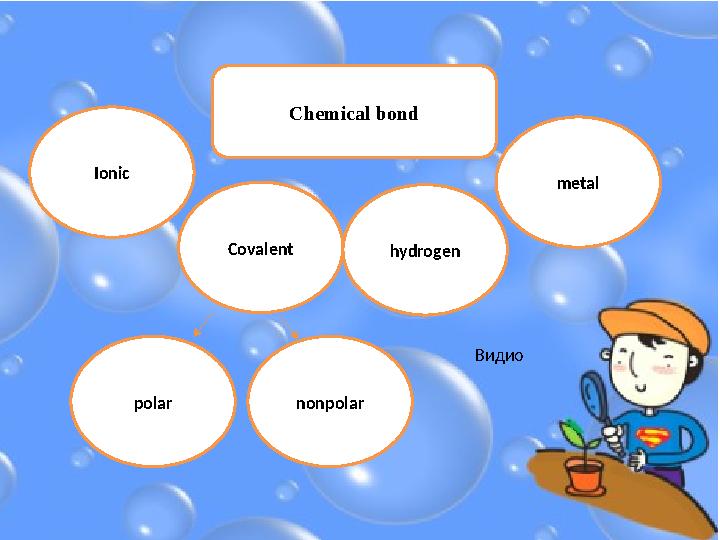
#10 слайд
Ionic
Covalent
polar nonpolar hydrogen metalС hemical bond
Видио
10 слайд
Ionic Covalent polar nonpolar hydrogen metalС hemical bond Видио
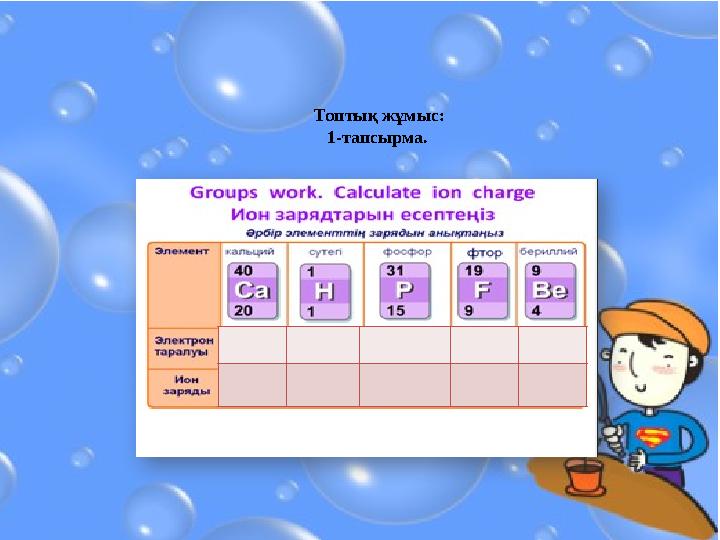
#11 слайд
Топтық жұмыс:
1-тапсырма.
11 слайд
Топтық жұмыс: 1-тапсырма.

#12 слайд
1. What is a compound?
2. What compounds are ionic compounds?
3. What compounds are с ovalent compounds?
4. What is the covalent polar bond? Assigning a lesson
Сабақты бекіту
12 слайд
1. What is a compound? 2. What compounds are ionic compounds? 3. What compounds are с ovalent compounds? 4. What is the covalent polar bond? Assigning a lesson Сабақты бекіту

#13 слайд
1.Сабақта қандай жаңа нәрселер
үйрендің?
What new things have you learned in
class?
2.Не қиын болды?
What was hard? Feedback
13 слайд
1.Сабақта қандай жаңа нәрселер үйрендің? What new things have you learned in class? 2.Не қиын болды? What was hard? Feedback

#14 слайд
Home task
Ережелерді жаттау
Understand definitions
Thank you
very much
14 слайд
Home task Ережелерді жаттау Understand definitions Thank you very much

шағым қалдыра аласыз