General Chemistry Safety and Laboratory Rules
Learn and observe the safety and laboratory rules!
1. DO NOT perform unauthorized experiments or work in a laboratory alone.
2. Approved eye protection must be worn at all times in the laboratory. Tennessee State law requires the use of such devices. Eye protection must be splash proof chemical goggles and be approved by your instructor. If you do get a chemical in your eye rinse immediately with large quantities of water using the eye-wash stations.
3. Long hair and loose clothing must be confined while in a laboratory.
4. Appropriate clothing must be worn at all times while in the laboratory. Your legs must be completely covered below the knee by your choice of clothing. If your clothing does not meet the requirement you may choose to wear an approved laboratory coat or apron which does cover your legs to your knees.
5. Closed shoes with socks must be worn at all times – open-toed shoes, backless shoes, sling backs, clogs, and sandals are not permitted.
6. Know the location and proper use of fire extinguishers, fire blankets, safety showers, eye wash devices, and first aid kits.
7. Before obtaining any chemicals carefully read the label on the reagent bottles.
8. Eating, smoking, and drinking are not allowed in a chemistry laboratory.
9. Thoroughly wash your hands after leaving the laboratory.
10. Mouth suction is never used to fill a pipette.
12. Never force glass tubing through cork or rubber stoppers without proper lubrication.
13. Never direct the open end of test tube toward yourself or anyone else.
14. Never pour water into concentrated acid.
15. Learn the proper procedure for igniting and operating a laboratory burner. Always extinguish the flame when the burner is not being used. Make sure that all flammable reagents are well removed before lighting the burner.
16. Liquid and solid waste containers must be properly used at all times.
17. Never place chemicals directly on the balance pan. Always use a proper weighing container when using a balance to weigh a chemical. Never pour chemicals directly over the balance.
18. Never return unused chemicals to their original container (unless directed to do so by the instructor).
19. Securely replace lids, caps, and stoppers after removing reagents from containers.
20. Always wipe spatulas clean before and after inserting into reagent bottles.
21. Report any accident and / or injury, however minor, to your instructor immediately.
22. Never place anything that is not directly required for the experiment on laboratory desks; other items may interfere with the experiment.
23. All personal belongings should be placed in the bookcases as you enter the laboratory.
24. Clean up any spill immediately.
25. Before leaving the laboratory, make sure your work area is clean and dry. Ensure that all gas, water, vacuum, and air valves are completely turned off.
26. Your instructor is available for any assistance you may need. Never hesitate to ask questions especially if there is any question concerning proper operating procedure. Be sure that you understand every instruction before proceeding.
Laboratory work № 1
Theme: Colored qualitative tests for amino acids and proteins
Аim: to teach students how to conduct qualitative reactions for amino acids and proteins.
Objectives: students must perform qualitative reactions and explain the results obtained.
For finding out proteins in biological materials and determination of their amino acid composition use the coloured reactions.
Experience 1. A biuret reaction is a quality reaction on peptid connections.
Substances, containing peptid connection in alkaline solution in presence CuSO4 form the connections painted in a violet color.
Reagents and materials: water solution of albumen, 2 % solution of sulfate of copper, ten percent solution of гидроксида of natrium.
Motion of work :
For experience take two test tubes. In one pour near one milliliter of solution of albumen, in other is the same amount of water. In both test tubes add an equal volume 10 % percent solution of caustic soda, 2-3 drops 2 % solution of blue vitriol. At shaking the blue -violet painting turns out in the first test tube. The results of experience and his explanation write down.
Experience 2. Xanthoproteic reaction.
Reagents and materials: water solution squirrel concentrated nitric.
Equipment: stand with test tubes, water bath, pipettes on 1 milliliter.
The process chemistry of reaction: a xanthoproteic reaction is characteristic for cyclic amino acids (Thyrosinum, phenylalanine and tryptophane), with a benzol ring, that an aquafortis forms the nitro-compound of yellow.
Motion of work :
In a test tube pour an about 1 milliliter of solution of albumen, 0,5 milliliter of the concentrated aquafortis and carefully heat on water bath. Solution is painted in yellow. The results of experience and his explanation write down.
Experience 3. Reaction of Schultz and Raspail.
Reagents and materials: solution of albumen, 10 % solution of saccharose, concentrated sulphuric acid.
The process chemistry of reaction: reaction of Schultz and Raspail, also characteristic for a tryptophane, consisting in appearance of the c.r. painting at cooperation of tryptophane with the hydroxymethylfurfural appearing as a result of hydrolysis of saccharose and dehydration of products of her hydrolysis of the concentrated sulphuric acid.
Motion of work :
In a test tube pour a 1 ml of solution of albumen, 1-2 drops 10 % solution of saccharose and carefully, on a wall, make in layers a pipette a 1 milliliter of the concentrated sulphuric acid. A c.r. ring appears on the border of two liquids. The results of experience and his explanation write down.
Laboratory work 2
Theme: The separation of the mixture by chromatographic distribution of amino acids on paper.
Aim: to teach students to carry out the separation of a mixture of amino acids on a paper chromatogram.
Objectives: students are expected to complete a paper chromatogram and explain the results obtained.
The theoretical part
Chromatography is a convenient and useful method for the separation of mixtures and for the identification of substances. The method has been especially valuable for the separation of closely related compounds. There are many different types of chromatography, but in this experiment we will illustrate the method with the separation of amino acids by paper chromatography.
We will be using four common amino acids: arginine, glutamic acid, leucine and valine. Each has a structure, which can be represented by where R represents the parts of the structure which are different with each amino acid. The two groups common to all amino acids are the -NH2 group (the amino group) and the -COOH group (the carboxylic acid group).
The basic procedure in this experiment consists of applying a small drop of the solution containing the substances to be separated near one end of a strip of absorbent paper. This end of the paper is then placed into a developing solvent, which flows upward along the paper by capillary action.
The degree of solubility of the components of the mixture in the solvent, as well as the degree of attraction of these components to the wet cellulose molecules in the paper fibers, will determine the distance that the solvent will carry each substance along the paper during a given time interval.
Those components that are quite soluble in the developing solvent, or that have a low affinity for cellulose, will be carried the greatest distance from the origin. The finished paper, with its spots, is called a chromatogram.
In this experiment, distances traveled by spots of various compounds will be compared on a single sheet of paper. It is a common procedure to measure the ratio of the distance the spot moves to the total distance the solvent front moves (beyond the original location of the spot) under the conditions of the experiment. This ratio is called the Rf value and is defined as:
Rf = distance the spot travels / distance the solvent travels
Tables of Rf values are published for many compounds under various conditions. Distances are measured to the center of the spots. When spots of unknown compounds are placed on the same chromatogram as spots of known materials it is often sufficient, under good experimental conditions, to compare distances from the starting line for knowns and unknowns. In simple cases, this is all that is required in order to identify the unknown.
The positions of colored substances on a chromatogram are easily detected by the human eye, while a colorless substance may be made visible by treatment with a reagent that converts it to a colored compound. For example, amino acids, which are all colorless by themselves, give a blue or purple color when they react with ninhydrin. Therefore, amino acids may be detected on a chromatogram by treatment with ninhydrin reagent. Other methods of detecting colorless materials on a chromatogram include the use of ultraviolet light to detect fluorescent compounds, or the use of a Geiger counter to detect radioactive samples.
In this experiment you will carry out paper chromatography in order to understand the basic principles of this separation technique. You will calculate the Rf values for known and unknown components of your amino acid solutions and use this information to identify the components of your unknown amino acid solution.
Safety Precautions
¨Wear gloves when using ninhydrin solvent
¨Use different toothpicks for each amino acid and unknowns so that cross contamination can be avoided.
¨Dispose of any left over developing solvent (butanol / acetic acid / water) in reclaim container provided. No solvent should be allowed to go down the drain.
¨Butanol is a flammable liquid. Make sure there are no open flames and/or heat near the developing solvent.
EXPERIMENTAL PROCEDURE
1. PREPARATION OF THE CHROMATOGRAPHIC PAPER
CAUTION: Wearing gloves, handle the paper (Whatman no. 1) only along the narrow edges.
Use paper towels to protect the paper from the desk surface and from your hands. Remember that your skin contains amino acids and therefore you must avoid any contact with the part of the paper to be used in the analysis. Take a rectangle of the paper that is cut (typically about 11 cm, x 20 cm) so that the with of the paper is just a little less than the depth of an 800 mL beaker and its length is enough to permit the formation of a cylinder that will fit inside of the beaker without touching its walls.
Still wearing your gloves, make a light pencil line 1.5 cm from one long edge. Place six equally spaced pencil marks along this line, keeping the end markings about 2.5 cm from the ends. Label the first four marks with the abbreviations of the four amino acid knowns, and the remaining two spots with the unknown numbers.
2. SETTING UP THE DEVELOPING SOLVENT
Use a clean dry 800 mL beaker and the developing solvent (butanol / acetic acid / water) from the supply in the HOOD, taking only what you need (only 30 mL). Record your observations on the properties of the solvent. Note also the location of the “reclaim bottle” in the HOOD that will be used for any unused or extra solvent at the end of the experiment. NO SOLVENT SHOULD BE ALLOWED TO GO DOWN THE DRAIN. Use a glass rod to add enough solvent to the beaker to cover the bottom to about 0.5 cm in depth. Do this without splashing the sides of the beaker with the solvent.
3. RUNNING THE CHROMATOGRAPHIC SEPARATION
Coil the spotted paper into a cylinder and fasten the two short ends together with stapler. Do not overlap the ends of the paper. A gap of one or two millimeters space between paper edges is helpful. Set the paper cylinder, spotted edge down, into the beaker containing the solvent solution.
The pencil line with the spots must be above the liquid level. The paper should not touch the sides of the beaker. Cover the beaker with plastic wrap and leave the paper cylinder in the beaker until the solvent has nearly reached the top edge of the paper. NOTE: Once you set your chromatogram inside the beaker with the solvent, do not move or agitate the beaker while the solvent is flowing upward. Any slight movement of the beaker will cause the components (the spots) to shift positions. Keep an eye on the level of the solvent in the beaker so that the chromatogram does not go dry during the process. Record the total time for the chromatographic process.
4. ANALYSIS OF THE CHROMATOGRAM
Remove the paper from the beaker, lay it flat on a clean paper towel and immediately mark the solvent front with a pencil. Allow the paper to dry. Take the paper to the HOOD and spray it with ninhydrin solution
Laboratory work № 3
Theme: The sedimentation reaction on proteins
Aim: to study the reactions of sedimentation of proteins under the action of various reagents.
Objective: To consolidate knowledge of the proteins’ primary structure and its role in formation of three-dimensional structure of the molecule. To form the notion of conformational states of a protein molecule and their significance in protein functioning. To acquaint with the usage of protein denaturation in medical practice.
Problems for discussion
1. Secondary, supersecondary, tertiary, quaternary structures of a protein molecule (concept, varieties and bonds stabilizing the structure).
2. Conformational changes in functioning of proteins. Interaction of proteins with ligands.
3. Denaturation. Reversibility of denaturation. Mechanisms of denaturing factors action.
4. General physical and chemical properties of proteins (viscosity of solutions, light diffusion, optical activity, mobility in the electric field, solubility in water).
5. Stability of protein solutions.
6. Sedimentation of proteins.
Practical part
Laboratory work 1. Ammonium sulfate precipitation (“salting-out” of proteins)
“Salting-out” is a reversible reaction of protein sedimentation from the solution by high concentrations of neutral salts: NaCl, (NH4)2SO4, MgSO4.
In presence of high salt concentrations dehydration of protein molecules and partial elimination of their charge take place. A number of factors affect the process of salting-out: hydrophylity of the protein, its relative molecular mass, its charge. That is why various concentrations of the same salt are needed for precipitation of various proteins. Albumins make precipitates in a saturated solution of (NH4)2SO4 and globulins — in a semi-saturated solution of (NH4)2SO4, because globulins have a high molecular mass and a smaller charge than those of albumins.
Salting-out of proteins is a reversible reaction as the protein deposit can be dissolved again when the salt concentration is reduced by dialysis or water dilution. The process of proteins deposition by NaCl is not as active as by ammonia sulfate due to a weaker hydration ability that is characterized by the position of ions in Hoffmeister’s series:
SO42– > Cl– > Br– > NO3– > I– > CNS–⎯
Ca2+ > Li+ > Na+ > K+ > Rb+ > Cs+
Separation of albumins and globulins of egg-white
Accomplishment. Add 20 drops of a saturated solution of (NH4)2SO4 to 20 drops of egg-white and carefully stir. Watch the egg-globulin precipitation. Leave for 5 minutes, then filter out the deposit using a paper filter. The filtrate still have another protein - egg-albumin. Add the fine powder of ammonia sulfate to the filtrate till complete saturation, i. e. till a new portion of the powder stays unsolved. Then filter out the albumin deposit. Expose the filtrate to biuret reaction: add 2 drops of 1 % solution of CuSO4 + 5 drops of 10 % solution of NaOH to the filtrate. A negative biuret reaction (blue staining) indicates to the absence of protein in the tested solution.
Conclusion:
Laboratory work 2. Irreversible sedimentation of proteins
Denaturation gives irreversible sedimentation of the protein. Denaturation results in breaking the protein native structure and its loss of biological properties, including solubility. In such reactions proteins suffer deep changes and cannot be solved in the primary diluter. Irreversible reactions include: protein precipitation by salts of heavy metals, by mineral and organic acids, alkaloid reagents and sedimentation while boiling.
Protein sedimentation by salts of heavy metals, unlike salting-out, occurs in low salt concentrations. Proteins interacting with salts of heavy metals (lead, copper, silver, mercury etc.) adsorb them forming salt-like and complex compounds soluble in the excess of these salts (excluding the salts of silver nitrate and mercury chloride), but insoluble in water. Dissolution of the precipitate in the excess of salts is called adsorption peptisation. It occurs as a result of acquiring the same positive charge by protein particles.
Accomplishment
|
Reagents |
1st test-tube |
2nd test-tube |
|
Egg-white solution |
5 drops |
5 drops |
|
1 % copper sulfate solution |
1–2 drops |
– |
|
5 % silver nitrate solution |
– |
1–2 drops |
|
Mark the formed precipitate |
||
|
1 % copper sulfate solution (excess) |
5-10 drops |
– |
|
5 % silver nitrate solution (excess) |
– |
5-10 drops |
|
Mark the dissolution of the precipitate |
||
Laboratory work № 4
Theme: Dialysis of protein solutions
Aim: to teach students to carry out dialysis of proteins.
Objectives: students are expected to complete the dialysis of proteins and explain the results obtained.
Method of dialysis based on different abilities of the components of a solution by diffusion through a thin film membrane having a selective permeability. The membrane is a porous film through pores which can penetrate small molecules. Method of dialysis is used to purify high-molecular compounds from low molecular weight and concentration of polymer solutions.
Reagents: aqueous protein solution (solution prepared as specified in the laboratory work № 1); a saturated solution of sodium chloride; a 0.5 % silver nitrate solution; a 10 % solution of nitric acid; 10 percent solution of sodium hydroxide; 1 % copper sulfate solution.
Equipment: tube, plastic bag; glass.
Progress
Task 1. Dialysis
In a test tube, mix equal volumes of protein solution and a saturated solution of sodium chloride. Pour the prepared solution of protein in a bag made of cellophane, filling it halfway. The bag hang on a glass rod and dip it into the beaker with distilled water. Sodium ions and chloride ions freely diffuse through the wall of the bag and evenly distributed throughout the volume of water. Protein molecules are larger than the pore sizes of cellophane, and remain in the bag. The dialysis is carried out at room temperature for 20 min.
Task 2. Analysis of the water in the glass
To 1 cm3 of liquid from a glass, add 2 drops of nitric acid solution and 2-3 drops of silver nitrate solution. Appears white precipitate of silver chloride. To 1 cm3 of liquid from a glass, add 5 drops of alkali solution and 1-2 drops of copper sulfate solution. Purple colour, typical for proteins.
Task 3. Analysis of the contents of the bag.
To 5 drops of solution from the bag, add 5 drops of alkali solution and 1-2 drops of copper sulfate solution. There is a characteristic staining caused by the formation of complex salts of copper with the polypeptide.
Registration of results:
Briefly describe the progress of the work. Make a schematic drawing of dialysis. Make a conclusion about the distribution of low and high molecular weight substances before and after dialysis.
Laboratory work № 5
Theme: Total nitrogen determination by the Kjeldahl
Aim: to teach students how to conduct quantitative determination of protein in biological material.
Objectives: students must perform a quantitative determination to explain the results and answer the test questions.
Method for the determination of nitrogen in the biological materials and foods Kjeldahl proposed in 1883 and still remains , as compared to other methods , the most accurate. This method determines the negatively charged trivalent organic nitrogen compounds and mineral .
PRINCIPLE OF THE METHOD. A portion of the analyzed material is boiled with concentrated sulfuric acid. In this organic carbon is oxidized to carbon dioxide, hydrogen - up water; nitrogen (trivalent negatively charged) is converted to ammonia and sulfuric acid which is taken in excess, it forms ammonium sulfate. Mineralized sample then basified with ammonia distilled off in an acid solution which was determined by titration.
Nitrate and nitrite nitrogen, heterocyclic compounds kernels during this process an ammonium salt not converted.
PROGRESS.
1. mineralization. Material for the study weighed on an analytical balance accurate to 0.0002 grams and is made without touching the edges to the bottom of the Kjeldahl flask (Figure 1) the sample for analysis should contain 10-20 milligrams of nitrogen.
From this weighed : the dry plant material - 300-500 mg of dry animal material - 100-200 mg , blood, organs and tissues of animals - 0.5-1.0 grams of milk - 2-4 grams whey - 10 -20 grams. To the flask was added 5-10 ml of concentrated sulfuric acid (density 1.84 ) , 5-7 grains of copper sulfate (catalyst ) , 2-4 grams of sodium or potassium sulphate ( to raise the boiling point ) and stirred in a circular motion.
The flask was then put in an inclined position on the heater placed in a fume cupboard, and close its glass hole hollow sleeve (special air cooler). To reduce foaming and accelerate mineralization immediately after the start of heating the flask was carefully added 2-3 milliliters of hydrogen peroxide. In the process of combustion of hydrogen peroxide is added 2-3 times, but not before the flask was removed from heat and allowed to stand at room temperature for 3-5 minutes. Mineralization is considered complete when the contents of the flask remains clear by boiling for 10-15 minutes after each addition of hydrogen peroxide.
In parallel with the experimental control sample put with the same reagents , but without the test material. The chemistry of this stage can be expressed by the following equations :
Biological material + H2SO4 CO2 +H2O + NH3
2 NH3 +H2SO4 = (NH4)2 SO4.
2. Distillation ammonia. This part of the work carried out in the stripping machine (Figure 2) , consisting of the distillation flask 1 , Kjeldahl nozzle 2 , a funnel with a glass tube 3 , the refrigerator 4 , a tube with a ball for protection against the suction of liquid 5 , a receiver or a glass bulb 6 .
Upon completion of mineralization with the contents of the Kjeldahl flask is left at room temperature for cooling and collecting device for stripping ammonia. Then, in the receiving beaker (or flask) comes with 25 ml solution mass concentration of 4 % boric acid and 20 ml of a solution containing sulfuric acid 0.05 mol / l (or salt = 0.1 mole / l), 3-5 drops of the mixed indicator (having a sharp color transition at pH 5,4 by the blue-violet in acidic medium to green in alkaline) and places it so that the lower end of the tube attached to the refrigerator, was immersed in the acid solution receiving flask.
Cooled mineralizat diluted 2-3 fold with water and transferred to a Kjeldahl flask of the distillation flask. Kjeldahl flask was rinsed 3-4 times with 20-30 ml water , washout was poured into a distillation flask. When rinsing take into account that the total volume of liquid in the distillation flask was about 1/3 of its capacity . The distillation flask make 3-5 drops indicator Tashiro , throw a few pieces of pumice or short glass tubes for even boil and put on the heater. All parts of the stripping apparatus is connected hermetically , is poured through a funnel to the contents of the distillation flask a solution with a mass fraction of 40 % sodium hydroxide until strongly alkaline reaction. When the contents of the flask turn green. The required amount of added alkali can be pre- calculated by the neutralization reaction.
Turn refrigerator and heating the contents of the distillation flask to the boil , distilled ammonia. End ammonia stripping determined by the absence of color change red litmus paper when applied to its liquid droplets flowing from the refrigerator, after disconnecting it from the tube with the ball. First check the completeness of the stripping of ammonia is carried out in 10-15 minutes after full warm eliminator. Typically, the distillation is complete after the increase in the receiving flask fluid volume is 2.5-3 times the initial value.
By the same procedure produces distillation flask contents control. The reactions of the second stage can be expressed as the following equations:
1. In the distillation flask:
(NH4)2SO4 = NaOH + Na2SO4 + H2O + NH3 .
2. In the receiving flask :
a) for capturing ammonia is distilled sulfuric acid -
2NH3 + H2SO4 = (NH4)2SO4,
b) for trapping ammonia is distilled solution of boric acid -
4H3BO3 + 2NH3 = (NH4)2B4O7 + 5H2O .
Resulting from the hydrolysis of ammonium tetraborate gives an alkaline reaction and the contents of the receiving flask painted in green.
3.Titration and calculation. After distilling off the ammonia refrigerator closure is lifted so that the free end of the tube with the ball is above the surface of the liquid receiving tube of the flask and the inside and outside are rinsed with distilled water. The contents of the receiving flask (distillate) is titrated with acid or base , depending on the compound used to absorb ammonia. When stripping the ammonia in the boric acid solution was titrated with a solution of distillate at a concentration of hydrochloric acid 0.1 mol / l ( sulfuric acid 0.05 mol / l ) until a clear green transition in the blue- purple ( color intermediate gray tones) . The reactions that occur during the titration can be described by the following equations :
(NH4)2B4O7 +2HCl + 5H2O = 2NH4Cl + 4H3BO3
(NH4)2B4O7 + H2SO4 + 5H2O = (NH4)2SO4 + 4H3BO3
From these equations can be easily calculated that 1 ml of hydrochloric acid at a concentration of 0.1 mol / l ( sulfuric acid 0.05 mol / l ) corresponds to 0.0014 grams of nitrogen .
Mass fraction of nitrogen in the material (X % ) is calculated as follows :

a - the volume of solution to the concentration of hydrochloric acid 0.1 mol / l (sulfuric acid = 0.05 moles / L) consumed for the titration test sample, mL ;
b - the volume of solution of the same acid consumed for the titration of the control sample, mL;
T - titer applied for the experience of hydrochloric (sulfuric acid);
m - mass of the test substance,
When stripping the ammonia in a solution of sulfuric (hydrochloric) acid distillate solution is titrated with a sodium hydroxide concentration (less potassium) 0.1 mol / L to move clear blue-purple to green. In this case, the excess solution is titrated with hydrochloric acid concentration of 0.1 mol / l (sulfuric - 0.05 mol / L) was taken for the absorption of ammonia. Mass fraction of nitrogen in the material calculated by the above formula, with the only difference that the designations "a", "b" and "T" are not to acid and alkali multiplying the weight proportion of nitrogen of 6.25 (conversion factor protein nitrogen ) content found in the material "raw" protein. "Raw" protein , in this case called because the calculation does not come from protein nitrogen and total nitrogen from a biological object (or food) as defined by the Kjeldahl method.

Fig. 2. Kjeldahl flask ( 1) and air cooler (2 )

![]()
Laboratory work № 6
Theme: Physical-chemical properties of enzymes.
Aim: to teach students the determination of the physical and chemical properties of enzymes.
Objectives: the students should undertake laboratory work, to explain the results and answer the test questions.
Purpose – to study the basic physical-chemical properties of enzymes.
Preparation of solutions of enzymes
Amylase, maltose (preparation of diluted saliva). Rinse mouth 2-3 times with water to remove food residue. Cylinder measure out 50 ml of distilled water and rinse it in mouth for 3-5 minutes. The collected liquid (50 ml) was filtered through cotton wool and the filtrate used for the work.
Hydrolysis of starch under the action of salivary amylase.
Starch is a high molecular polysaccharide, the most important carbohydrate in the human diet, found in flour products, cereals, potatoes. Starch can be broken down into glucose by boiling with concentrated mineral acids – sulfuric, hydrochloric (inorganic catalysts). Hydrolysis of starch occurs through the stage of formation of dextrins – shallow products of amylolysis. Starch forms with iodine a blue coloration, dextrin (depending on their complexity) – purple, reddish-brown or yellow-brown. The absence of color indicates the formation of maltose or glucose.
In starch there is almost no free aldehyde groups, so it does not give characteristic for these groups, the reaction with Fehling's reagent. Glucose and maltose have a free aldehyde group and give this reaction. The saliva enzyme amylase hydrolyzes starch to dextrin and disaccharide maltose. Saliva contains the enzyme maltase that breaks down maltose to glucose. The number of free aldehyde groups will increase, consequently decreasing characteristic of these groups feling reaction, resulting in the observed red staining. Thus, the hydrolysis of starch can be judged on the basis of two reactions: – reaction for starch with iodine; the reaction with Fehling's reagent to aldehyde group of glucose:
Reagents and materials: 1 % starch paste; amylase solution; 1 % solution of iodine in potassium iodide; the solution of the Fehling's.
The order of execution of work
In two test tubes pour 2 ml of the starch paste in one of them 2 ml of water, and in another 2 ml of saliva. Both test tubes with glass rods, immersed in them, are simultaneously placed in a water bath at 40 °C. After 4 min from each tube selected with the help of glass rod a drop of liquid and mix them individually with a drop of iodine, pre-applied to the wafer. Repeat the sampling after 6 and l0 min. Painting with iodine samples from tubes containing saliva, changing from blue to blue-purple, brownish red, red and finally yellow. The contents of the test tube with amylase added is 0.5–0.8 ml of Fehling's solution and heated until boiling. Forms a red precipitate of copper oxide (I) due to restoration of the hydroxide of copper (II) formed by the low molecular weight maltose and dextrin.
The control sample under the same conditions does not restore copper hydroxide (II) to copper oxide (I).
Laboratory work № 7
Тheme: The study of the properties of enzymes.
Aim: to study the influence of some factors on the enzyme activity.
Objectives: to perform experiments, explain the results and draw conclusions.
Comparison of the action of inorganic catalysts and enzymes
Reagents: 1 % starch solution, 10 % hydrochloric acid; the solution of iodine in potassium iodide; Fehling's solution; solution of saliva.
The order of execution of work. In three test tubes pour 5 ml of starch solution. The first tube was added I ml of distilled water, second I ml of hydrochloric acid, in the third – 1 ml of dilute saliva, containing amylase. After mixing, the tubes 1 and 3 are placed in a water bath at 38 ºC and the tube 2 in a boiling water bath. After 15-20 minutes take out the test tube from the water bath, cooled and from each take 2 samples for the subsequent determination of starch and glucose: the first is by reaction with iodine, the reaction with Fehling's reagent. For determination of glucose in each test tube take 3 ml of the solution add 2 ml of Fehling's reagent and heated. The appearance of red or orange precipitate of copper oxide (I) indicates the presence of glucose.
The test results are entered in a table.
Specificity of enzymes action
This is the most important property of enzymes, the main reason is the complementary nature of structure of the active enzyme with the substrate structure. Each enzyme works only on a single substance or a group of complex substances. For example, amylase accelerates the hydrolysis of starch, lipase accelerates the hydrolysis of fats. Despite the fact that we are dealing with the process of hydrolysis, amylase cannot replace the lipase and Vice versa. The specificity of action on a given substrate depends on the presence of certain groups in the configuration of the substrate.
Reagents: 1% solution of sucrose; 1% starch solution; Fehling's solution; saliva diluted 10 times.
The order of execution of work. In one test tube pour 1 ml of sucrose solution, 1 ml of starch solution. In both test tubes add 1 ml of diluted saliva and put them in a water bath with a temperature of 37 °C for 10 min. After heating both test tubes with Fehling's reagent in the presence of glucose. With sucrose, the reaction must be negative.
Influence of activators and inhibitors on enzyme activity
A great influence on the activity of enzymes has the presence in solution of a number of chemical compounds. Some of them boost the activity of enzymes (activators), and others are depressing (inhibitors). To many activators include sodium, molybdenum, magnesium, manganese, cobalt. Of inhibitors of enzymes known heavy metal ions, salts of hydrocyanic, acid monoiodotyrosine and so on.
Reagents: a solution of urease; a 1 % solution of lead acetate; 1 % solution of copper sulphate; 0.25 % solution of urea; phenolphthalein.
The order of execution of work. In two test tubes pour 2 ml of urease solution. In one of them add 0,5 ml of a solution of lead acetate, and 0.5 ml of copper sulfate solution, and in both tubes, I ml of urea solution and 2 drops of phenolphthalein. Crimson, the color does not appear due to the loss of the urease enzymatic activity.
Laboratory work 8
Theme: The discovery of vitamins qualitative responses.
Aim: to study the qualitative reactions to vitamins.
Objectives: to perform experiments, explain the results and draw conclusions.
Reaction to vitamin B1 (thiamine). Vitamin B1 when heated with red blood salt (K3[Fe(CN)6]) is oxidized to thiochrome with yellow color.
In 2 test-tube, pour 5-10 drops of 10 % NaOH solution and the same 5 % solution (K3[Fe(CN)6]). In one of the tubes add about 1 ml of vitamin B1, to another the same amount of water. Heat both test tubes. The first fluid is colored in orange-yellow, the second (control) light yellow.
The results for the response record.
Response to vitamin B2 (Riboflavin). When restoring vitamin B2 in an acidic environment is formed intermediate connection red (roboflavin), which then turns into a colorless compound (lackieren).
In test tube pour about 1 ml of vitamin B2, add a few drops of concentrated hydrochloric acid and dip a piece of zinc metal. There is a rapid release of hydrogen bubbles. The liquid gradually turns pink and then fades.
The results for the response record.
Reactions to vitamin PP (nicotinic acid). During heating of vitamin e with acetate of copper, a precipitate of copper salt of nicotinic acid.
In a test tube place a small amount of vitamin e, add 10-20 drops of 10 % solution of acetic acid and heat to dissolve the vitamin. To the hot solution add an equal volume of 5 % solution of copper acetate. When standing, the precipitate appears blue.
The results for the response record.
Reaction to vitamin C (ascorbic acid). The reaction is based on the regenerating properties of vitamin C is Easily oxidized to dehydroascorbic acid, vitamin restores various dyes (methylene blue, dichlofenthion), which in this case are discolored.
In 2 test-tube pour 1 ml of methylene blue solution and add 5 drops of 10 % soda solution. In one of the tubes add a few drops of solution of vitamin C. the other (control) an equal amount of water. Heat both test tubes. In tubes containing vitamin C, the liquid becomes colourless.
The results for the response record.
Reactions to vitamin a (retinol). Vitamin a in the presence of sulfuric acid with chloroform gives a red coloration. In a dry test tube pour a few drops of fish oil and the same of chloroform. Carefully add 3-5 drops of concentrated sulfuric acid. The liquid becomes red. The results for the response record.
Reaction to vitamin D (calciferol).
A) Vitamin D in the presence of hydrochloric acid gives with aniline red staining. In a dry test tube, pour about 1 ml of fish oil and the same mixture of aniline with hydrochloric acid. Stir the contents of the tubes. Carefully heat. The liquid gradually acquires a red color.
B) Vitamin D a solution of bromine in chloroform gives a greenish-blue coloration. In dry test tube put approximately 1 ml of fish oil and the same amount of bromine in chloroform. The liquid is a greenish-blue color.
The results for the response record.
Reaction to the vitamin E (tocopherol). The oxidation of vitamin E nitric acid forms a substance, colored red. In a dry test-tube pour 1 ml of tocopherol solution, add 5 drops of concentrated nitric acid, and shake gently heat the contents of the tube. Red emulsion is formed. The results for the response record.
Laboratory work № 9
Theme: The allocation of ribonucleoproteins from yeast and detection of hydrolysis products of quality responses.
Aim: to study the hydrolysis of RNA and qualitative reactions to the hydrolysis products.
Objectives: to perform experiments, explain the results and draw conclusions.
The selection of the nucleoproteins from a biological material can be accomplished by various methods: by extraction with water or a mild to medium alkaline solutions and salts, followed by precipitation with acetic acid nucleoproteins, ultracentrifugation, gelfiltration. When applying any of them you first need to destroy the cell membrane. The material taken for the study, homogenized. All nucleoproteins can be subjected to hydrolytic decomposition, in which there is a consistent gap first essential, and then the glycosidic linkages. First, the nucleoproteins break down into simple proteins and nucleic acids. Proteins can then be subjected to hydrolysis to peptides and amino acids.
THE PRACTICAL PART
The allocation of ribonucleoproteins
Progress
To obtain ribonucleoproteins in a mortar were placed 10 g of yeast. Add 4 ml water-ether mixture (1:1) and stirred. Then add 5 g of quartz sand and triturated, priliva small portions of 50 ml of 0.4 % NaOH. The resulting mass is triturated for at least 15 minutes, after which the suspension was filtered through a paper filter or centrifuge. Throws the resulting precipitate, and the filtrate (centrifugate) are collected, placed in a beaker and gradually add a 10% solution of acetic acid (5-6 ml). After each addition check the litmus test of the reaction medium. Acid is added until, until the reaction medium becomes slightly acid. In these circumstances, the nucleoproteins precipitates. The resulting suspension is centrifuged, the filtrate is decanted and the precipitate is separated nucleoproteins.
The hydrolysis of nucleoproteins yeast.
To study the chemical composition of nucleoproteins is carried out acid hydrolysis of yeast, rich in nucleoproteins, and identify the products of hydrolysis of the polypeptides, purine bases, carbohydrates and phosphoric acid specific for each substance reactions.
Progress
In a large wide tube (15 х 1,5 cm) was placed 0.5 g of fresh baking or 0.1 g of dry yeast and pour 4 ml of 10% sulfuric acid solution. The tube is closed by a plug, a fridge in which is a glass tube with a length of 25-30 cm, and put on an asbestos net.
1 hour after the start of boiling of the liquid hydrolysis is stopped, allow the contents to cool, filtered and in the filtrate determine the products of hydrolysis of nucleoproteins.
Qualitative reactions for the hydrolysis products of nucleoproteins
Determination of proteins and peptides
When conducting hydrolysis under the conditions set out in clause 4.2, proteins are subject to him only partially and only broken down to peptides. Proteins and peptides in the hydrolysate detected using biuret reaction.
Progress
The biuret reaction to the polypeptides. To 5 drops of hydrolysate, add 10 drops of 10 % solution NaOH and one drop of a 1% solution of copper sulfate. The liquid turns purple.
The opening of purine bases. Silver test for purine bases. 10 drops of hydrolysate is neutralized with one drop of ammonia and add 5 drops of 1% solution of silver nitrate. After 3-5 minutes falls loose brown precipitate of the silver compounds of purine bases (adenine, guanine).
The opening of the pentoses. Detection of pentoses is based on their dehydration concentrated acids to form furfural, which gives with thymol (reaction MOLISA), α-naphthol and orcine colored compounds.
Attention ! These reactions are carried out only with dry test tubes. Ribose and deoxyribose can be opened by reaction with diphenylamine, which with ribose gives a green coloration, and with the deoxyribose gives a blue coloration.
The Reaction Of MOLISA
Progress
Qualitative reaction of MOLISA on ribose and deoxyribose. To the 10 drops of hydrolysate yeast add 2-3 drops of 1 % alcoholic solution of thymol or α-naphthol, mix and on the side of the tube gently pour 20 drops of concentrated sulfuric acid. By shaking the test tube appears red staining due to the formation of the condensation products of furfural with thymol or α-naphthol.
In test tube pour 10 drops of hydrolysate, add 1-2 drops of a 0.1 % solution of α-naphthol and mix well. Then carefully, on the side of the tube, pour 10-15 drops of concentrated sulfuric acid. When standing on the boundary between two phases there is a purple staining.
In test tube pour 10 drops of hydrolysate, add 10-15 drops of orcine and 10-15 drops of concentrated sulfuric acid. The mixture was heated to boiling and observe for staining (green or pink-red).
Opening of the ribose and desoxyribose
Progress
Test Trommer on ribose and desoxyribose. To 5 drops of hydrolysate, add 10 drops of 30 % solution of sodium hydroxide and 1-3 drops of 7 % solution of copper sulfate until a persistent precipitate copper hydroxide. The liquid was stirred and heated to boiling. The result falls a red precipitate of cuprous oxide, or yellow precipitate of hydrate of copper oxide due to oxidation of the ribose and the restoration of the hydroxide of copper in the hydrate of copper oxide.
The detection of phosphoric acid
Progress
The molybdenum test. To 20 drops of molybdenic reagent (solution of ammonium molybdate in nitric acid) add 2-3 drops of hydrolysate and boil for a few minutes on the fire. In the presence of phosphoric acid, the liquid is painted in lemon yellow color. Upon cooling a yellow crystalline precipitate falls complex compounds of phosphorus-ammonium molybdate (NH4)3PO4 x 12MoO3.
The results are entered in table 1:
The test results table 1.
|
The name of the protein complex |
Prosthetic group |
Name reactions that detect components of proteid |
Used reagents, the reaction conditions |
What caused the reaction |
|
|
|
|
|
|
In test tube pour 10 drops of hydrolysate and gradually add concentrated ammonia solution until the sharp smell, and then 10 drops of magnesia mixture. Formed a crystalline precipitate of magnesium phosphate-ammonium MgNH4PO4.
REPORTING
The report shall be prepared stating the purpose, tasks, includes the equations of reactions, experimental data and conclusions.
Laboratory work 10
Theme: Allocation of dezoxiribonukleoproteid from a spleen.
Aim: to obtain DNA from spleen.
Objectives: to perform experiments, explain the results and draw conclusions.
With nucleoproteids the liver, a spleen, a pancreas, kidneys, yeast are rich. They are dissolved in the diluted solutions of alkalis and drop out in a deposit at solution acidulation. Dezoxiribonukleoproteida are well dissolved also in salt solutions.
Reactants and materials: a) a liver or a spleen of cattle or a pig, fresh or frozen; b) chloride sodium, 5 percentage solution; c) a wooden stick with notches.
Experience performance order: 2-2,5 g of a liver or a spleen cut on small slices and then pound in a mortar with the 5th percentage solution of chloride sodium, having added a little glass powder. Solution of salt is added in the small portions (on 10-15 ml), all about 80 ml spend it.
Pound 12-15 minutes before obtaining homogeneous weight. Contents of a mortar are spilled in centrifugal test tubes and centrifuged 10-15 minutes (at 2500 revolutions per minute) then measure the volume of a tsentrifugat (merging it in the glass cylinder),
In a glass pour the distilled water (which volume has to be six times more than the volume of a centrifugat) and, slowly stirring slowly in a glass with a wooden stick, pour in water centrifugat. Dezoxiribonukleoproteida drop out in the form of threads which reel up on a stick.
High-quality reaction to DNA
Existence of DNA is determined by color reactions, characteristic for a dezoksiriboza. Often apply reaction with biphenyl amine (C6H5-NH-C6H5). Biphenyl amine with dezoxiribozy or DNA forms connection of blue color. The ribose and RNA give green coloring with biphenyl amine.
Reactants and materials: a) a deposit of a dezoxiribonukleoproteid (you watch the previous work); b) difenilaminovy reactant: 1 g of biphenyl amine dissolve in 100 ml of ice acetic acid. Add 2,75 g to solution the concentrated chamois of acid (density 1,836); c) the caustic rubbed, 0,4 percentage solution.
Experience performance order: The part of a deposit of a dezoxiribonukleoproteid is transferred to a test tube and add 0,5-1 ml of solution of a caustic natr (before dissolution). To solution flow the equal volume of a difenilaminovy reactant. The deposit which is dropping out in the beginning will be dissolved next portions of a reactant then it is narevat within 15-20 minutes in the boiling water bath. There is a blue coloring.
Laboratory work № 11
Theme: High-quality reactions to difficult proteins
Aim: studying of high-quality reactions to difficult proteins.
Objectives: to perform experiments, explain the results and draw conclusions.
Relevance of a subject: At soft hydrolysis there is rather superficial disintegration of protein. Pirimidin nucleotides at such hydrolysis don't break up, and purin break up with education the purin of the bases (adenine and a guanine), a ribose and phosphoric acid. Carrying out hydrolysis under certain conditions, it is possible to allocate consistently various intermediate products of hydrolytic disintegration of nucleoproteids and to define them by high-quality reactions.
High-quality reactions to proteins and peptides
When carrying out hydrolysis under the conditions specified in laboratory work 9, proteins are exposed to it only partially and are split only to peptides. Proteins and peptides in a hydrolyzate find by means of biuret reaction.
Progress
The biuret reaction to the polypeptides. To 5 drops of hydrolysate, add 10 drops of 10 % solution NaOH and one drop of a 1% solution of copper sulfate. The liquid turns purple.
The opening of purine bases. Silver test for purine bases. 10 drops of hydrolysate is neutralized with one drop of ammonia and add 5 drops of 1 % solution of silver nitrate. After 3-5 minutes falls loose brown precipitate of the silver compounds of purine bases (adenine, guanine).
The opening of the pentoses. Detection of pentoses is based on their dehydration concentrated acids to form furfural, which gives with thymol (reaction MOLISA), α-naphthol and orcine colored compounds.
Attention ! These reactions are carried out only with dry test tubes. Ribose and deoxyribose can be opened by reaction with diphenylamine, which with ribose gives a green coloration, and with the deoxyribose gives a blue coloration.
The Reaction Of MOLISA
Progress
Qualitative reaction of MOLISA on ribose and dezoxyribose. To the 10 drops of hydrolysate yeast add 2-3 drops of 1 % alcoholic solution of thymol or α-naphthol, mix and on the side of the tube gently pour 20 drops of concentrated sulfuric acid. By shaking the test tube appears red staining due to the formation of the condensation products of furfural with thymol or α-naphthol.
In test tube pour 10 drops of hydrolysate, add 1-2 drops of a 0.1 % solution of α-naphthol and mix well. Then carefully, on the side of the tube, pour 10-15 drops of concentrated sulfuric acid. When standing on the boundary between two phases there is a purple staining.
In test tube pour 10 drops of hydrolysate, add 10-15 drops of orcine and 10-15 drops of concentrated sulfuric acid. The mixture was heated to boiling and observe for staining (green or pink-red).
Opening of the ribose and deoxyribose
Progress
Test Trommer on ribose and deoxyribose. To 5 drops of hydrolysate, add 10 drops of 30 % solution of sodium hydroxide and 1-3 drops of 7 % solution of copper sulfate until a persistent precipitate copper hydroxide. The liquid was stirred and heated to boiling. The result falls a red precipitate of cuprous oxide, or yellow precipitate of hydrate of copper oxide due to oxidation of the ribose and the restoration of the hydroxide of copper in the hydrate of copper oxide.
The detection of phosphoric acid
Progress
The molybdenum test. To 20 drops of molybdenic reagent (solution of ammonium molybdate in nitric acid) add 2-3 drops of hydrolysate and boil for a few minutes on the fire. In the presence of phosphoric acid, the liquid is painted in lemon yellow color. Upon cooling a yellow crystalline precipitate falls complex compounds of phosphorus-ammonium molybdate (NH4)3PO4 ∙ 12MoO3.
The results are entered in table 2:
The test results table 2.
|
The name of the protein complex |
Prosthetic group |
Name reactions that detect components of proteid |
Used reagents, the reaction conditions |
What caused the reaction |
|
|
|
|
|
|
|
|
|
|
|
|
In test tube pour 10 drops of hydrolysate and gradually add concentrated ammonia solution until the sharp smell, and then 10 drops of magnesia mixture. Formed a crystalline precipitate of magnesium phosphate-ammonium MgNH4PO4.
Laboratoty work № 12
Тhеme: Qualitative reactions for carbohydrates
Aim: to study the qualitative reactions for carbohydrates.
Objectives: to perform experiments, explain the results and draw conclusions.
1. Qualitative reactions for glucose.
A) Reaction of silver mirror. In test tube pour 2 ml of glucose solution, add half as ammonia solution of silver nitrate and boil out the lamp. On the side of the tube, the mirror is formed as a result of the separation of metallic silver.
The test results and the equation of the reaction is recorded.
B) Reaction of Felling. In test tube pour 2 ml of glucose solution and add an equal amount of Felling's reagent consisting of equal parts of solutions 1 (CuSO4) and 2 (NaOH) containing tartrate sodium-potassium, contributing to the dissolution of the precipitate of hydrate of copper oxide. Boil out the lamp. Appears brick-red staining resulting from copper oxide.
The test results and the equation of the reaction is recorded.
B) Reaction оf Nylander. In test tube pour the same amount of glucose solution, as in the previous experiments, and add 5-6 drops of Nylander's reagent (consists of basic bismuth nitrate, caustic soda, and the salt sеgnet; in the interaction of the first two reagents is formed a hydrate of bismuth oxide, held in solution the salt sеgnet). Boil for 2-3 minutes. The contents of the tube turns black due to the separation of metallic bismuth.
The test results and the equation of the reaction is recorded.
2. The Selivanov's reaction to fructose.
In test tube pour 2 ml of a solution of fructose. Add 5 drops of Selivanov's reagent (0.5 g resorcinol in 100 ml of 20 % HCl solution) and boil out the lamp. Becomes cherry-red colouring.
The test results and the equation of the reaction is recorded.
3. The reaction of Tollens on the pentose.
In test tube pour 2 ml of a solution of any pentose, add an equal volume of 20 % HCl solution and 5 drops of 0.5 % solution floroglûcina. Boil for 1 minute. As a result of interaction of the formed furfural with phloroglucinol appears red staining.
In the absence of pentoses can be used to experience wood sawdust containing pentosans (polysaccharides, built of pentoses). In this case, use a solution of concentrated hydrochloric acid. When this occurs the hydrolysis of the pentosans. Then add a few crystals of floroglûcina, and after a few minutes, there is a red staining.
4. Reducing properties of disaccharides.
3 the test tube is poured 2 ml of a solution of disaccharides: the first is sucrose, the second – maltose, in the third – lactose. With all three produce the reaction of Felling (as described above).
The test results and the equation of the reaction is recorded.
5. The opening of fructose in the composition of the disaccharides.
As in the previous experience, it is poured into a test tube solutions of sucrose, maltose and lactose. With all three solutions produce Selivanov's reaction.
The test results and the equation of the reaction is recorded.
6. Acid hydrolysis of sucrose.
In test tube pour 4 ml of the sucrose solution, add 1 ml of a 1 normal solution of sulphuric acid and boil on a spirit lamp for 2 minutes. The solution was neutralized with 1 ml of 2 normal sodium hydroxide solution, add an equal volume of Felling's reagent and boil the lamp.
The test results and the equation of the reaction is recorded.
7. Qualitative reactions for polysaccharides.
In one test tube put 1 ml of starch solution, and in the other the same amount of a solution of glycogen in each and add 1-2 drops of Lugol's solution (iodine solution in potassium iodide). See the coloration.
The test results and the equation of the reaction is recorded.
8. The precipitation of the glycogen with alcohol.
Glycogen in water forms a colloidal solution, which is hydrophilic Sol. Therefore, a substance that takes water means violate its stability.
In test tube pour 1 ml of glycogen solution and add 10 ml of alcohol. The solution immediately became cloudy, and after some time a solid precipitate.
The test results and the equation of the reaction is recorded.
Laboratory work № 13
Theme: Separation of lipids from muscle tissue and quality detection
Aim: to obtain lipids from the biological material and to study qualitative reaction.
Objectives: to perform experiments, explain the results and draw conclusions.
Methods of extraction of fat.
Typically, the lipids extracted from dried (dehydrated) tissues relevant organic solvents (alcohols, ethers, benzene, toluene, gasoline, acetone, pyridine, chloroform, carbon tetrachloride, carbon disulfide, petroleum ether, etc.). For the separation of lipids using their different solubility in various solvents: some of these are soluble in ether but poorly in acetone (e.g., phospholipids), the other soluble in benzene, but insoluble in alcohol (cholesterol, cerebroside), etc. back fat (oil) is extracted easily. To retrieve the related lipids only after the destruction of protein-lipid complexes. Lipids from the bound state to a free state is transferred either through the use gidrolizuemye funds, or by prior boiling of the material with alcohol. In the latter case, the lipids are excreted in unchanged form.
The most simple method of determination of total lipids in the tissues is the method of long insisting hinge-plate fabrics in chloroform - methanol mixture. On a difference of weights of sample before and after extraction found percentage lipids.
In the allocation of lipids from biological materials is their oxidation and degradation, leading to the formation of by-products. Therefore, the selection of lipids should be carried out quickly, in conditions excluding the influence of factors such as increased temperature, oxygen, light, contamination by trace metals, etc.
For the extraction using mixture of methanol and chloroform, which destroys lipoprotein complexes and thus gives an opportunity to fully extract the lipids.
Qualitative reaction for fats and oils.
The drop of oil on the hourly or slide add one drop of a 1% solution of osmium acid. The oil turns black. In addition to the osmium acid, as a reagent on the oil used the dye Sudan III, which stains oil in different shades of red.
For the qualitative detection of oil products in the tissue sections (plant or animal), moistened with one of these reagents and observed under the microscope: a drop of oil in the tissues are colored in the corresponding color (black or osmium acid in red - from the Sudan III).
Detection of glycerol in fats (aсroleina sample)
In test tube add 2-3 drops of oil (fat) and add five times the amount of anhydrous potassium hydrogen sulfate. Heat the test tube gently but strongly (in fume cupboard) until dense white fumes. Note (carefully!) the sharp irritating odor of acrolein. If these couples add a piece of filter paper dampened with ammonia solution of silver oxide, the paper will turn black. Filter paper moistened with a solution faxencerie acid, approached to the neck of the tube, is gradually painted in a pinkish-purple color.
You can repeat this experience with wax instead of oil. The acrolein in this case are not formed.
Akroleina test performed to detect lipid solubility of glycerol. By heating glycerol in the presence of substances that take away water means (a hydrous sulfate of potash, magnesium sulfate, boric acid) the formation of an acrylic unsaturated aldehyde - acrolein:
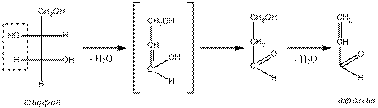
The definition of saturation (unsaturation) of fats.
Unsaturation of the fat depends on the presence in its composition of unsaturated fatty acids. Unsaturated compounds, as is well known, easy to enter into the reactions of addition, oxidation and polymerization. Usually the degree of unsaturation determine the iodine number. Iodine number is measured by the number of grams of iodine that is attached to 100 grams of fat.
To determine the iodine number of the titrated solution of the fat in chloroform (0,5 grams of fat in 3 ml of chloroform) of 0,001 n solution of iodine in chloroform until a distinct pink color.
Laboratory work № 14
Тhеme: Determination of properties of lipids.
Aim: to study the properties of lipids.
Objectives: to perform experiments, explain the results and draw conclusions.
Qualitative tests for fats. The formation of oil stains. A drop of oil applied with a glass rod on a piece of paper. Spot is formed, does not disappear when heated.
The solubility of fats.
Reagents: a) vegetable oil (sunflower, flax, cotton or other); b) tallow (mutton, beef); c) dietil ether; d) acetone, d) ethanol; e) distilled water.
Put two rows of 4 tubes each. In the tubes of the first row: put a few drops of vegetable oil; in the tubes of the second row – piece of solid fat. In the first test tube of each row pour 2 ml of distilled water, the second poured the same amount of diethyl ether, then poured acetone in a fourth pour of alcohol. All tubes are shaken and see the fat solubility in various solvents. Swab with alcohol, it is recommended to heat in a water bath. Record the results of experience.
Emulsification of fatty oils.
Reagents: a) vegetable oil; b) sodium carbonate, 2 % solution; b) soap, 2 % solution; d) bile; d) distilled water.
In four test-tubes put 5 drops of oil. In the first test tube add 2 ml of distilled water in another test tube add 2 ml of 2% restora sodium carbonate (soda), in the third test tube add the same 2 % solution of soap, in the fourth test tube add 2 ml of water and a few drops of bile. All tubes are shaken and observe the formation in the first test tube unstable emulsion of oil in water, rapidly exfoliating when standing, while others see the formation of stable emulsions due to the action of added emulsifiers, which are adsorbed in the outer layer of fat caped and lower their surface tension.
Aсrolein reaction.
Using sample to determine the presence of acrolein of glycerol in fats. When heating fat with potassium acid sulfate (KHSO4), sodium acid sulfate (NaHSO4) or boric acid (H3BO3) cleavage occurs from a molecule of glycerol with two molecules of water and the formation of acrylic aldehyde, or acrolein, having a pungent irritating odor (burnt fat):
Reagents: a) vegetable oil or animal fat; b) beeswax; C) acid sulfate or potassium acid sulfate sodium crystalline; g) boric acid, crystalline.

In dry test tube add a few drops of vegetable oil or a piece of animal fat, add a little acid powder potassium sulfate or boric acid and gently heated. See white vapours of acrolein, having a pungent odor. Repeat the reaction with the wax – acrolein is not formed, since glycerin is not included in the composition of the waxes.
Laboratory work № 15
Theme: The secretion of cholesterol from the brain. Qualitative detection of cholesterol.
Aim: To be able to determine the cholesterol concentration in blood serum and to interpret this index, to determine the concentration of ketone bodies in urine and interpret the result.
Professional orientation of students:
The increase of ketone bodies contents in blood and in urine is the index of fat catabolismin activation in an organism and insufficient use of ketone bodies as alternative energetic substrate in tissues. This is the reason of development of ketosis at starvation, diabetes mellitus, thyrotoxicosis etc.
The contents of phospholipids in blood is the important index of functional state of liver, possibilities of transport and metabolism of cholesterol. The increase of cholesterol in blood occurs in the prevailing of animal fats and carbohydrates in a diet, as well as in smoking, alcoholism etc.
Methodology of Practical Class.
I. Cholesterol.
Work 1. Qualitative detection and quantitative determination of ketone bodies in urine
1. The qualitative detection of acetone (Legal reaction).
Acetone and acetoacetic acid with sodium nitroprussicum in alkaline medium form red colored compounds. In adding of the concentrated acetic acid the cherry-red complex salt is formed.
Materials and reagents. Urine containing ketone bodies, normal urine, 10 % solution of sodium nitroprussicum, concentrated acetic acid, 10 % solution of NaOH.
Protocol.
Add into two tubes on 0,5 ml of urine: into the 1-st tube – urine of healthy person; into the 2-nd – urine containing ketone bodies. Into both tubes add 0,5 ml of 10 % solution NaOH and 5-7 drops of 10 % solution of sodium nitroprussicum. Observe the appearance of red color in the 2-nd tube. Add several drops of concentrated acetic acid. Red color is changed to cherry color.
2. The qualitative detection of acetone (Herhardt reaction).
In adding of FeCl3 solution to urine containing ketone bodies the sediment is formed.
Materials and reagents. Urine consisting ketone bodies, normal urine, 10 % solution FeCl3.
Protocol.
Add into two tubes 2 ml of urine: into the 1-st tube – urine of healthy person; into the 2-nd – urine containing ketone bodies. Into both tubes add several drops of 10 % solution FeCl3. Observe the formation of sediment.
The quantitative determination of ketone bodies in urine.
If urine contains acetone, the violet ring is formed in the border between layer of ammonia and urine containing sodium nitroprussicum and concentrated acetic acid. The velocity of ring appearance depends on the concentration of acetone in urine. The appearance of ring between 3 and 4 min means that concentration of acetone is 0,0085 g/l.
Materials and reagents. Urine containing ketone bodies, 50 % solution of ammonia sulfate, 10 % solution of sodium nitroprussicum, concentrated acetic acid.
Protocol.
Take 8 tubes. Add into each tube (except the first one) 1 ml of water. Into the 1st and 2nd tubes add 1 ml of urine. Thus, in the 1-st tube the urine is not diluted and in the 2-nd one – two fold diluted. Replace 1 ml of mixture from the 2-nd tube into the 3-rd one, from the 3-rd into the 4-th and so on. Remove 1 ml of mixture from the last tube. Thus, the dilution in the series of tubes will be in 2, 4, 8 and etc. times. Add into each tube 8 drops of ammonia sulfate, 8 drops of 10 % solution of sodium nitroprussicum and 8 drops of concentrated acetic acid. Mix the content of each tube. Carefully (don’t mix) along the walls of tubes pour 1 ml of concentrated ammonia. Note the tube where the violet ring between layers of liquids appears between 3 and 4 min.
Calculation.
Use the formula:
X = 8,5 x a x 1,5,
where
X – concentration of acetone in urine (mg/day);
a – dilution of urine.
Work 1. Qualitative detection of cholesterol.
Materials and reagents. The solution of cholesterol in chloroform; concentrated sulfuric acid; acetic anhydride.
Protocol.
Add into the dry tube 1 ml of chloroform solution of cholesterol, 5-6 drops of acetic anhydride and 1-2 drops of concentrated sulfuric acid. Mix the sample carefully. Observe the appearance of green color.
Work 2. The quantitative determination of cholesterol in blood serum.
Cholesterol in the presence of acetic anhydride and mixture of acetic and sulfuric acid form the green colored compound. The cholesterol concentration is proportional to the intensity of color.
Materials and reagents. Blood serum, reagent N1 (mixture of acetic acid, acetic anhydride and sulfuric acid – 1:5:1), standard solution of cholesterol.
Protocol.
Add into two dry tubes 2 ml of reagent N1. Add into 1-st tube 0,1 ml of standard solution of cholesterol and into 2-nd – 0,1 ml of blood serum. Stir up the tubes and stay in darkness for 20 min. The green color appears. Colorimeter the samples using red filter and water as control.
Calculation:
X = Eexp / Est x Cst (mmol/l).
Eexp – extinction of sample containing blood plasma;
Est - extinction of sample containing standard solution;
Cst – cholesterol concentration in standard solution.
25
жүктеу мүмкіндігіне ие боласыз
Бұл материал сайт қолданушысы жариялаған. Материалдың ішінде жазылған барлық ақпаратқа жауапкершілікті жариялаған қолданушы жауап береді. Ұстаз тілегі тек ақпаратты таратуға қолдау көрсетеді. Егер материал сіздің авторлық құқығыңызды бұзған болса немесе басқа да себептермен сайттан өшіру керек деп ойласаңыз осында жазыңыз
Тақырыбы: Амин қышқылдары мен ақуыздарға арналған түрлі-түсті сапалық сынақтар
Тақырыбы: Амин қышқылдары мен ақуыздарға арналған түрлі-түсті сапалық сынақтар
General Chemistry Safety and Laboratory Rules
Learn and observe the safety and laboratory rules!
1. DO NOT perform unauthorized experiments or work in a laboratory alone.
2. Approved eye protection must be worn at all times in the laboratory. Tennessee State law requires the use of such devices. Eye protection must be splash proof chemical goggles and be approved by your instructor. If you do get a chemical in your eye rinse immediately with large quantities of water using the eye-wash stations.
3. Long hair and loose clothing must be confined while in a laboratory.
4. Appropriate clothing must be worn at all times while in the laboratory. Your legs must be completely covered below the knee by your choice of clothing. If your clothing does not meet the requirement you may choose to wear an approved laboratory coat or apron which does cover your legs to your knees.
5. Closed shoes with socks must be worn at all times – open-toed shoes, backless shoes, sling backs, clogs, and sandals are not permitted.
6. Know the location and proper use of fire extinguishers, fire blankets, safety showers, eye wash devices, and first aid kits.
7. Before obtaining any chemicals carefully read the label on the reagent bottles.
8. Eating, smoking, and drinking are not allowed in a chemistry laboratory.
9. Thoroughly wash your hands after leaving the laboratory.
10. Mouth suction is never used to fill a pipette.
12. Never force glass tubing through cork or rubber stoppers without proper lubrication.
13. Never direct the open end of test tube toward yourself or anyone else.
14. Never pour water into concentrated acid.
15. Learn the proper procedure for igniting and operating a laboratory burner. Always extinguish the flame when the burner is not being used. Make sure that all flammable reagents are well removed before lighting the burner.
16. Liquid and solid waste containers must be properly used at all times.
17. Never place chemicals directly on the balance pan. Always use a proper weighing container when using a balance to weigh a chemical. Never pour chemicals directly over the balance.
18. Never return unused chemicals to their original container (unless directed to do so by the instructor).
19. Securely replace lids, caps, and stoppers after removing reagents from containers.
20. Always wipe spatulas clean before and after inserting into reagent bottles.
21. Report any accident and / or injury, however minor, to your instructor immediately.
22. Never place anything that is not directly required for the experiment on laboratory desks; other items may interfere with the experiment.
23. All personal belongings should be placed in the bookcases as you enter the laboratory.
24. Clean up any spill immediately.
25. Before leaving the laboratory, make sure your work area is clean and dry. Ensure that all gas, water, vacuum, and air valves are completely turned off.
26. Your instructor is available for any assistance you may need. Never hesitate to ask questions especially if there is any question concerning proper operating procedure. Be sure that you understand every instruction before proceeding.
Laboratory work № 1
Theme: Colored qualitative tests for amino acids and proteins
Аim: to teach students how to conduct qualitative reactions for amino acids and proteins.
Objectives: students must perform qualitative reactions and explain the results obtained.
For finding out proteins in biological materials and determination of their amino acid composition use the coloured reactions.
Experience 1. A biuret reaction is a quality reaction on peptid connections.
Substances, containing peptid connection in alkaline solution in presence CuSO4 form the connections painted in a violet color.
Reagents and materials: water solution of albumen, 2 % solution of sulfate of copper, ten percent solution of гидроксида of natrium.
Motion of work :
For experience take two test tubes. In one pour near one milliliter of solution of albumen, in other is the same amount of water. In both test tubes add an equal volume 10 % percent solution of caustic soda, 2-3 drops 2 % solution of blue vitriol. At shaking the blue -violet painting turns out in the first test tube. The results of experience and his explanation write down.
Experience 2. Xanthoproteic reaction.
Reagents and materials: water solution squirrel concentrated nitric.
Equipment: stand with test tubes, water bath, pipettes on 1 milliliter.
The process chemistry of reaction: a xanthoproteic reaction is characteristic for cyclic amino acids (Thyrosinum, phenylalanine and tryptophane), with a benzol ring, that an aquafortis forms the nitro-compound of yellow.
Motion of work :
In a test tube pour an about 1 milliliter of solution of albumen, 0,5 milliliter of the concentrated aquafortis and carefully heat on water bath. Solution is painted in yellow. The results of experience and his explanation write down.
Experience 3. Reaction of Schultz and Raspail.
Reagents and materials: solution of albumen, 10 % solution of saccharose, concentrated sulphuric acid.
The process chemistry of reaction: reaction of Schultz and Raspail, also characteristic for a tryptophane, consisting in appearance of the c.r. painting at cooperation of tryptophane with the hydroxymethylfurfural appearing as a result of hydrolysis of saccharose and dehydration of products of her hydrolysis of the concentrated sulphuric acid.
Motion of work :
In a test tube pour a 1 ml of solution of albumen, 1-2 drops 10 % solution of saccharose and carefully, on a wall, make in layers a pipette a 1 milliliter of the concentrated sulphuric acid. A c.r. ring appears on the border of two liquids. The results of experience and his explanation write down.
Laboratory work 2
Theme: The separation of the mixture by chromatographic distribution of amino acids on paper.
Aim: to teach students to carry out the separation of a mixture of amino acids on a paper chromatogram.
Objectives: students are expected to complete a paper chromatogram and explain the results obtained.
The theoretical part
Chromatography is a convenient and useful method for the separation of mixtures and for the identification of substances. The method has been especially valuable for the separation of closely related compounds. There are many different types of chromatography, but in this experiment we will illustrate the method with the separation of amino acids by paper chromatography.
We will be using four common amino acids: arginine, glutamic acid, leucine and valine. Each has a structure, which can be represented by where R represents the parts of the structure which are different with each amino acid. The two groups common to all amino acids are the -NH2 group (the amino group) and the -COOH group (the carboxylic acid group).
The basic procedure in this experiment consists of applying a small drop of the solution containing the substances to be separated near one end of a strip of absorbent paper. This end of the paper is then placed into a developing solvent, which flows upward along the paper by capillary action.
The degree of solubility of the components of the mixture in the solvent, as well as the degree of attraction of these components to the wet cellulose molecules in the paper fibers, will determine the distance that the solvent will carry each substance along the paper during a given time interval.
Those components that are quite soluble in the developing solvent, or that have a low affinity for cellulose, will be carried the greatest distance from the origin. The finished paper, with its spots, is called a chromatogram.
In this experiment, distances traveled by spots of various compounds will be compared on a single sheet of paper. It is a common procedure to measure the ratio of the distance the spot moves to the total distance the solvent front moves (beyond the original location of the spot) under the conditions of the experiment. This ratio is called the Rf value and is defined as:
Rf = distance the spot travels / distance the solvent travels
Tables of Rf values are published for many compounds under various conditions. Distances are measured to the center of the spots. When spots of unknown compounds are placed on the same chromatogram as spots of known materials it is often sufficient, under good experimental conditions, to compare distances from the starting line for knowns and unknowns. In simple cases, this is all that is required in order to identify the unknown.
The positions of colored substances on a chromatogram are easily detected by the human eye, while a colorless substance may be made visible by treatment with a reagent that converts it to a colored compound. For example, amino acids, which are all colorless by themselves, give a blue or purple color when they react with ninhydrin. Therefore, amino acids may be detected on a chromatogram by treatment with ninhydrin reagent. Other methods of detecting colorless materials on a chromatogram include the use of ultraviolet light to detect fluorescent compounds, or the use of a Geiger counter to detect radioactive samples.
In this experiment you will carry out paper chromatography in order to understand the basic principles of this separation technique. You will calculate the Rf values for known and unknown components of your amino acid solutions and use this information to identify the components of your unknown amino acid solution.
Safety Precautions
¨Wear gloves when using ninhydrin solvent
¨Use different toothpicks for each amino acid and unknowns so that cross contamination can be avoided.
¨Dispose of any left over developing solvent (butanol / acetic acid / water) in reclaim container provided. No solvent should be allowed to go down the drain.
¨Butanol is a flammable liquid. Make sure there are no open flames and/or heat near the developing solvent.
EXPERIMENTAL PROCEDURE
1. PREPARATION OF THE CHROMATOGRAPHIC PAPER
CAUTION: Wearing gloves, handle the paper (Whatman no. 1) only along the narrow edges.
Use paper towels to protect the paper from the desk surface and from your hands. Remember that your skin contains amino acids and therefore you must avoid any contact with the part of the paper to be used in the analysis. Take a rectangle of the paper that is cut (typically about 11 cm, x 20 cm) so that the with of the paper is just a little less than the depth of an 800 mL beaker and its length is enough to permit the formation of a cylinder that will fit inside of the beaker without touching its walls.
Still wearing your gloves, make a light pencil line 1.5 cm from one long edge. Place six equally spaced pencil marks along this line, keeping the end markings about 2.5 cm from the ends. Label the first four marks with the abbreviations of the four amino acid knowns, and the remaining two spots with the unknown numbers.
2. SETTING UP THE DEVELOPING SOLVENT
Use a clean dry 800 mL beaker and the developing solvent (butanol / acetic acid / water) from the supply in the HOOD, taking only what you need (only 30 mL). Record your observations on the properties of the solvent. Note also the location of the “reclaim bottle” in the HOOD that will be used for any unused or extra solvent at the end of the experiment. NO SOLVENT SHOULD BE ALLOWED TO GO DOWN THE DRAIN. Use a glass rod to add enough solvent to the beaker to cover the bottom to about 0.5 cm in depth. Do this without splashing the sides of the beaker with the solvent.
3. RUNNING THE CHROMATOGRAPHIC SEPARATION
Coil the spotted paper into a cylinder and fasten the two short ends together with stapler. Do not overlap the ends of the paper. A gap of one or two millimeters space between paper edges is helpful. Set the paper cylinder, spotted edge down, into the beaker containing the solvent solution.
The pencil line with the spots must be above the liquid level. The paper should not touch the sides of the beaker. Cover the beaker with plastic wrap and leave the paper cylinder in the beaker until the solvent has nearly reached the top edge of the paper. NOTE: Once you set your chromatogram inside the beaker with the solvent, do not move or agitate the beaker while the solvent is flowing upward. Any slight movement of the beaker will cause the components (the spots) to shift positions. Keep an eye on the level of the solvent in the beaker so that the chromatogram does not go dry during the process. Record the total time for the chromatographic process.
4. ANALYSIS OF THE CHROMATOGRAM
Remove the paper from the beaker, lay it flat on a clean paper towel and immediately mark the solvent front with a pencil. Allow the paper to dry. Take the paper to the HOOD and spray it with ninhydrin solution
Laboratory work № 3
Theme: The sedimentation reaction on proteins
Aim: to study the reactions of sedimentation of proteins under the action of various reagents.
Objective: To consolidate knowledge of the proteins’ primary structure and its role in formation of three-dimensional structure of the molecule. To form the notion of conformational states of a protein molecule and their significance in protein functioning. To acquaint with the usage of protein denaturation in medical practice.
Problems for discussion
1. Secondary, supersecondary, tertiary, quaternary structures of a protein molecule (concept, varieties and bonds stabilizing the structure).
2. Conformational changes in functioning of proteins. Interaction of proteins with ligands.
3. Denaturation. Reversibility of denaturation. Mechanisms of denaturing factors action.
4. General physical and chemical properties of proteins (viscosity of solutions, light diffusion, optical activity, mobility in the electric field, solubility in water).
5. Stability of protein solutions.
6. Sedimentation of proteins.
Practical part
Laboratory work 1. Ammonium sulfate precipitation (“salting-out” of proteins)
“Salting-out” is a reversible reaction of protein sedimentation from the solution by high concentrations of neutral salts: NaCl, (NH4)2SO4, MgSO4.
In presence of high salt concentrations dehydration of protein molecules and partial elimination of their charge take place. A number of factors affect the process of salting-out: hydrophylity of the protein, its relative molecular mass, its charge. That is why various concentrations of the same salt are needed for precipitation of various proteins. Albumins make precipitates in a saturated solution of (NH4)2SO4 and globulins — in a semi-saturated solution of (NH4)2SO4, because globulins have a high molecular mass and a smaller charge than those of albumins.
Salting-out of proteins is a reversible reaction as the protein deposit can be dissolved again when the salt concentration is reduced by dialysis or water dilution. The process of proteins deposition by NaCl is not as active as by ammonia sulfate due to a weaker hydration ability that is characterized by the position of ions in Hoffmeister’s series:
SO42– > Cl– > Br– > NO3– > I– > CNS–⎯
Ca2+ > Li+ > Na+ > K+ > Rb+ > Cs+
Separation of albumins and globulins of egg-white
Accomplishment. Add 20 drops of a saturated solution of (NH4)2SO4 to 20 drops of egg-white and carefully stir. Watch the egg-globulin precipitation. Leave for 5 minutes, then filter out the deposit using a paper filter. The filtrate still have another protein - egg-albumin. Add the fine powder of ammonia sulfate to the filtrate till complete saturation, i. e. till a new portion of the powder stays unsolved. Then filter out the albumin deposit. Expose the filtrate to biuret reaction: add 2 drops of 1 % solution of CuSO4 + 5 drops of 10 % solution of NaOH to the filtrate. A negative biuret reaction (blue staining) indicates to the absence of protein in the tested solution.
Conclusion:
Laboratory work 2. Irreversible sedimentation of proteins
Denaturation gives irreversible sedimentation of the protein. Denaturation results in breaking the protein native structure and its loss of biological properties, including solubility. In such reactions proteins suffer deep changes and cannot be solved in the primary diluter. Irreversible reactions include: protein precipitation by salts of heavy metals, by mineral and organic acids, alkaloid reagents and sedimentation while boiling.
Protein sedimentation by salts of heavy metals, unlike salting-out, occurs in low salt concentrations. Proteins interacting with salts of heavy metals (lead, copper, silver, mercury etc.) adsorb them forming salt-like and complex compounds soluble in the excess of these salts (excluding the salts of silver nitrate and mercury chloride), but insoluble in water. Dissolution of the precipitate in the excess of salts is called adsorption peptisation. It occurs as a result of acquiring the same positive charge by protein particles.
Accomplishment
|
Reagents |
1st test-tube |
2nd test-tube |
|
Egg-white solution |
5 drops |
5 drops |
|
1 % copper sulfate solution |
1–2 drops |
– |
|
5 % silver nitrate solution |
– |
1–2 drops |
|
Mark the formed precipitate |
||
|
1 % copper sulfate solution (excess) |
5-10 drops |
– |
|
5 % silver nitrate solution (excess) |
– |
5-10 drops |
|
Mark the dissolution of the precipitate |
||
Laboratory work № 4
Theme: Dialysis of protein solutions
Aim: to teach students to carry out dialysis of proteins.
Objectives: students are expected to complete the dialysis of proteins and explain the results obtained.
Method of dialysis based on different abilities of the components of a solution by diffusion through a thin film membrane having a selective permeability. The membrane is a porous film through pores which can penetrate small molecules. Method of dialysis is used to purify high-molecular compounds from low molecular weight and concentration of polymer solutions.
Reagents: aqueous protein solution (solution prepared as specified in the laboratory work № 1); a saturated solution of sodium chloride; a 0.5 % silver nitrate solution; a 10 % solution of nitric acid; 10 percent solution of sodium hydroxide; 1 % copper sulfate solution.
Equipment: tube, plastic bag; glass.
Progress
Task 1. Dialysis
In a test tube, mix equal volumes of protein solution and a saturated solution of sodium chloride. Pour the prepared solution of protein in a bag made of cellophane, filling it halfway. The bag hang on a glass rod and dip it into the beaker with distilled water. Sodium ions and chloride ions freely diffuse through the wall of the bag and evenly distributed throughout the volume of water. Protein molecules are larger than the pore sizes of cellophane, and remain in the bag. The dialysis is carried out at room temperature for 20 min.
Task 2. Analysis of the water in the glass
To 1 cm3 of liquid from a glass, add 2 drops of nitric acid solution and 2-3 drops of silver nitrate solution. Appears white precipitate of silver chloride. To 1 cm3 of liquid from a glass, add 5 drops of alkali solution and 1-2 drops of copper sulfate solution. Purple colour, typical for proteins.
Task 3. Analysis of the contents of the bag.
To 5 drops of solution from the bag, add 5 drops of alkali solution and 1-2 drops of copper sulfate solution. There is a characteristic staining caused by the formation of complex salts of copper with the polypeptide.
Registration of results:
Briefly describe the progress of the work. Make a schematic drawing of dialysis. Make a conclusion about the distribution of low and high molecular weight substances before and after dialysis.
Laboratory work № 5
Theme: Total nitrogen determination by the Kjeldahl
Aim: to teach students how to conduct quantitative determination of protein in biological material.
Objectives: students must perform a quantitative determination to explain the results and answer the test questions.
Method for the determination of nitrogen in the biological materials and foods Kjeldahl proposed in 1883 and still remains , as compared to other methods , the most accurate. This method determines the negatively charged trivalent organic nitrogen compounds and mineral .
PRINCIPLE OF THE METHOD. A portion of the analyzed material is boiled with concentrated sulfuric acid. In this organic carbon is oxidized to carbon dioxide, hydrogen - up water; nitrogen (trivalent negatively charged) is converted to ammonia and sulfuric acid which is taken in excess, it forms ammonium sulfate. Mineralized sample then basified with ammonia distilled off in an acid solution which was determined by titration.
Nitrate and nitrite nitrogen, heterocyclic compounds kernels during this process an ammonium salt not converted.
PROGRESS.
1. mineralization. Material for the study weighed on an analytical balance accurate to 0.0002 grams and is made without touching the edges to the bottom of the Kjeldahl flask (Figure 1) the sample for analysis should contain 10-20 milligrams of nitrogen.
From this weighed : the dry plant material - 300-500 mg of dry animal material - 100-200 mg , blood, organs and tissues of animals - 0.5-1.0 grams of milk - 2-4 grams whey - 10 -20 grams. To the flask was added 5-10 ml of concentrated sulfuric acid (density 1.84 ) , 5-7 grains of copper sulfate (catalyst ) , 2-4 grams of sodium or potassium sulphate ( to raise the boiling point ) and stirred in a circular motion.
The flask was then put in an inclined position on the heater placed in a fume cupboard, and close its glass hole hollow sleeve (special air cooler). To reduce foaming and accelerate mineralization immediately after the start of heating the flask was carefully added 2-3 milliliters of hydrogen peroxide. In the process of combustion of hydrogen peroxide is added 2-3 times, but not before the flask was removed from heat and allowed to stand at room temperature for 3-5 minutes. Mineralization is considered complete when the contents of the flask remains clear by boiling for 10-15 minutes after each addition of hydrogen peroxide.
In parallel with the experimental control sample put with the same reagents , but without the test material. The chemistry of this stage can be expressed by the following equations :
Biological material + H2SO4 CO2 +H2O + NH3
2 NH3 +H2SO4 = (NH4)2 SO4.
2. Distillation ammonia. This part of the work carried out in the stripping machine (Figure 2) , consisting of the distillation flask 1 , Kjeldahl nozzle 2 , a funnel with a glass tube 3 , the refrigerator 4 , a tube with a ball for protection against the suction of liquid 5 , a receiver or a glass bulb 6 .
Upon completion of mineralization with the contents of the Kjeldahl flask is left at room temperature for cooling and collecting device for stripping ammonia. Then, in the receiving beaker (or flask) comes with 25 ml solution mass concentration of 4 % boric acid and 20 ml of a solution containing sulfuric acid 0.05 mol / l (or salt = 0.1 mole / l), 3-5 drops of the mixed indicator (having a sharp color transition at pH 5,4 by the blue-violet in acidic medium to green in alkaline) and places it so that the lower end of the tube attached to the refrigerator, was immersed in the acid solution receiving flask.
Cooled mineralizat diluted 2-3 fold with water and transferred to a Kjeldahl flask of the distillation flask. Kjeldahl flask was rinsed 3-4 times with 20-30 ml water , washout was poured into a distillation flask. When rinsing take into account that the total volume of liquid in the distillation flask was about 1/3 of its capacity . The distillation flask make 3-5 drops indicator Tashiro , throw a few pieces of pumice or short glass tubes for even boil and put on the heater. All parts of the stripping apparatus is connected hermetically , is poured through a funnel to the contents of the distillation flask a solution with a mass fraction of 40 % sodium hydroxide until strongly alkaline reaction. When the contents of the flask turn green. The required amount of added alkali can be pre- calculated by the neutralization reaction.
Turn refrigerator and heating the contents of the distillation flask to the boil , distilled ammonia. End ammonia stripping determined by the absence of color change red litmus paper when applied to its liquid droplets flowing from the refrigerator, after disconnecting it from the tube with the ball. First check the completeness of the stripping of ammonia is carried out in 10-15 minutes after full warm eliminator. Typically, the distillation is complete after the increase in the receiving flask fluid volume is 2.5-3 times the initial value.
By the same procedure produces distillation flask contents control. The reactions of the second stage can be expressed as the following equations:
1. In the distillation flask:
(NH4)2SO4 = NaOH + Na2SO4 + H2O + NH3 .
2. In the receiving flask :
a) for capturing ammonia is distilled sulfuric acid -
2NH3 + H2SO4 = (NH4)2SO4,
b) for trapping ammonia is distilled solution of boric acid -
4H3BO3 + 2NH3 = (NH4)2B4O7 + 5H2O .
Resulting from the hydrolysis of ammonium tetraborate gives an alkaline reaction and the contents of the receiving flask painted in green.
3.Titration and calculation. After distilling off the ammonia refrigerator closure is lifted so that the free end of the tube with the ball is above the surface of the liquid receiving tube of the flask and the inside and outside are rinsed with distilled water. The contents of the receiving flask (distillate) is titrated with acid or base , depending on the compound used to absorb ammonia. When stripping the ammonia in the boric acid solution was titrated with a solution of distillate at a concentration of hydrochloric acid 0.1 mol / l ( sulfuric acid 0.05 mol / l ) until a clear green transition in the blue- purple ( color intermediate gray tones) . The reactions that occur during the titration can be described by the following equations :
(NH4)2B4O7 +2HCl + 5H2O = 2NH4Cl + 4H3BO3
(NH4)2B4O7 + H2SO4 + 5H2O = (NH4)2SO4 + 4H3BO3
From these equations can be easily calculated that 1 ml of hydrochloric acid at a concentration of 0.1 mol / l ( sulfuric acid 0.05 mol / l ) corresponds to 0.0014 grams of nitrogen .
Mass fraction of nitrogen in the material (X % ) is calculated as follows :

a - the volume of solution to the concentration of hydrochloric acid 0.1 mol / l (sulfuric acid = 0.05 moles / L) consumed for the titration test sample, mL ;
b - the volume of solution of the same acid consumed for the titration of the control sample, mL;
T - titer applied for the experience of hydrochloric (sulfuric acid);
m - mass of the test substance,
When stripping the ammonia in a solution of sulfuric (hydrochloric) acid distillate solution is titrated with a sodium hydroxide concentration (less potassium) 0.1 mol / L to move clear blue-purple to green. In this case, the excess solution is titrated with hydrochloric acid concentration of 0.1 mol / l (sulfuric - 0.05 mol / L) was taken for the absorption of ammonia. Mass fraction of nitrogen in the material calculated by the above formula, with the only difference that the designations "a", "b" and "T" are not to acid and alkali multiplying the weight proportion of nitrogen of 6.25 (conversion factor protein nitrogen ) content found in the material "raw" protein. "Raw" protein , in this case called because the calculation does not come from protein nitrogen and total nitrogen from a biological object (or food) as defined by the Kjeldahl method.

Fig. 2. Kjeldahl flask ( 1) and air cooler (2 )

![]()
Laboratory work № 6
Theme: Physical-chemical properties of enzymes.
Aim: to teach students the determination of the physical and chemical properties of enzymes.
Objectives: the students should undertake laboratory work, to explain the results and answer the test questions.
Purpose – to study the basic physical-chemical properties of enzymes.
Preparation of solutions of enzymes
Amylase, maltose (preparation of diluted saliva). Rinse mouth 2-3 times with water to remove food residue. Cylinder measure out 50 ml of distilled water and rinse it in mouth for 3-5 minutes. The collected liquid (50 ml) was filtered through cotton wool and the filtrate used for the work.
Hydrolysis of starch under the action of salivary amylase.
Starch is a high molecular polysaccharide, the most important carbohydrate in the human diet, found in flour products, cereals, potatoes. Starch can be broken down into glucose by boiling with concentrated mineral acids – sulfuric, hydrochloric (inorganic catalysts). Hydrolysis of starch occurs through the stage of formation of dextrins – shallow products of amylolysis. Starch forms with iodine a blue coloration, dextrin (depending on their complexity) – purple, reddish-brown or yellow-brown. The absence of color indicates the formation of maltose or glucose.
In starch there is almost no free aldehyde groups, so it does not give characteristic for these groups, the reaction with Fehling's reagent. Glucose and maltose have a free aldehyde group and give this reaction. The saliva enzyme amylase hydrolyzes starch to dextrin and disaccharide maltose. Saliva contains the enzyme maltase that breaks down maltose to glucose. The number of free aldehyde groups will increase, consequently decreasing characteristic of these groups feling reaction, resulting in the observed red staining. Thus, the hydrolysis of starch can be judged on the basis of two reactions: – reaction for starch with iodine; the reaction with Fehling's reagent to aldehyde group of glucose:
Reagents and materials: 1 % starch paste; amylase solution; 1 % solution of iodine in potassium iodide; the solution of the Fehling's.
The order of execution of work
In two test tubes pour 2 ml of the starch paste in one of them 2 ml of water, and in another 2 ml of saliva. Both test tubes with glass rods, immersed in them, are simultaneously placed in a water bath at 40 °C. After 4 min from each tube selected with the help of glass rod a drop of liquid and mix them individually with a drop of iodine, pre-applied to the wafer. Repeat the sampling after 6 and l0 min. Painting with iodine samples from tubes containing saliva, changing from blue to blue-purple, brownish red, red and finally yellow. The contents of the test tube with amylase added is 0.5–0.8 ml of Fehling's solution and heated until boiling. Forms a red precipitate of copper oxide (I) due to restoration of the hydroxide of copper (II) formed by the low molecular weight maltose and dextrin.
The control sample under the same conditions does not restore copper hydroxide (II) to copper oxide (I).
Laboratory work № 7
Тheme: The study of the properties of enzymes.
Aim: to study the influence of some factors on the enzyme activity.
Objectives: to perform experiments, explain the results and draw conclusions.
Comparison of the action of inorganic catalysts and enzymes
Reagents: 1 % starch solution, 10 % hydrochloric acid; the solution of iodine in potassium iodide; Fehling's solution; solution of saliva.
The order of execution of work. In three test tubes pour 5 ml of starch solution. The first tube was added I ml of distilled water, second I ml of hydrochloric acid, in the third – 1 ml of dilute saliva, containing amylase. After mixing, the tubes 1 and 3 are placed in a water bath at 38 ºC and the tube 2 in a boiling water bath. After 15-20 minutes take out the test tube from the water bath, cooled and from each take 2 samples for the subsequent determination of starch and glucose: the first is by reaction with iodine, the reaction with Fehling's reagent. For determination of glucose in each test tube take 3 ml of the solution add 2 ml of Fehling's reagent and heated. The appearance of red or orange precipitate of copper oxide (I) indicates the presence of glucose.
The test results are entered in a table.
Specificity of enzymes action
This is the most important property of enzymes, the main reason is the complementary nature of structure of the active enzyme with the substrate structure. Each enzyme works only on a single substance or a group of complex substances. For example, amylase accelerates the hydrolysis of starch, lipase accelerates the hydrolysis of fats. Despite the fact that we are dealing with the process of hydrolysis, amylase cannot replace the lipase and Vice versa. The specificity of action on a given substrate depends on the presence of certain groups in the configuration of the substrate.
Reagents: 1% solution of sucrose; 1% starch solution; Fehling's solution; saliva diluted 10 times.
The order of execution of work. In one test tube pour 1 ml of sucrose solution, 1 ml of starch solution. In both test tubes add 1 ml of diluted saliva and put them in a water bath with a temperature of 37 °C for 10 min. After heating both test tubes with Fehling's reagent in the presence of glucose. With sucrose, the reaction must be negative.
Influence of activators and inhibitors on enzyme activity
A great influence on the activity of enzymes has the presence in solution of a number of chemical compounds. Some of them boost the activity of enzymes (activators), and others are depressing (inhibitors). To many activators include sodium, molybdenum, magnesium, manganese, cobalt. Of inhibitors of enzymes known heavy metal ions, salts of hydrocyanic, acid monoiodotyrosine and so on.
Reagents: a solution of urease; a 1 % solution of lead acetate; 1 % solution of copper sulphate; 0.25 % solution of urea; phenolphthalein.
The order of execution of work. In two test tubes pour 2 ml of urease solution. In one of them add 0,5 ml of a solution of lead acetate, and 0.5 ml of copper sulfate solution, and in both tubes, I ml of urea solution and 2 drops of phenolphthalein. Crimson, the color does not appear due to the loss of the urease enzymatic activity.
Laboratory work 8
Theme: The discovery of vitamins qualitative responses.
Aim: to study the qualitative reactions to vitamins.
Objectives: to perform experiments, explain the results and draw conclusions.
Reaction to vitamin B1 (thiamine). Vitamin B1 when heated with red blood salt (K3[Fe(CN)6]) is oxidized to thiochrome with yellow color.
In 2 test-tube, pour 5-10 drops of 10 % NaOH solution and the same 5 % solution (K3[Fe(CN)6]). In one of the tubes add about 1 ml of vitamin B1, to another the same amount of water. Heat both test tubes. The first fluid is colored in orange-yellow, the second (control) light yellow.
The results for the response record.
Response to vitamin B2 (Riboflavin). When restoring vitamin B2 in an acidic environment is formed intermediate connection red (roboflavin), which then turns into a colorless compound (lackieren).
In test tube pour about 1 ml of vitamin B2, add a few drops of concentrated hydrochloric acid and dip a piece of zinc metal. There is a rapid release of hydrogen bubbles. The liquid gradually turns pink and then fades.
The results for the response record.
Reactions to vitamin PP (nicotinic acid). During heating of vitamin e with acetate of copper, a precipitate of copper salt of nicotinic acid.
In a test tube place a small amount of vitamin e, add 10-20 drops of 10 % solution of acetic acid and heat to dissolve the vitamin. To the hot solution add an equal volume of 5 % solution of copper acetate. When standing, the precipitate appears blue.
The results for the response record.
Reaction to vitamin C (ascorbic acid). The reaction is based on the regenerating properties of vitamin C is Easily oxidized to dehydroascorbic acid, vitamin restores various dyes (methylene blue, dichlofenthion), which in this case are discolored.
In 2 test-tube pour 1 ml of methylene blue solution and add 5 drops of 10 % soda solution. In one of the tubes add a few drops of solution of vitamin C. the other (control) an equal amount of water. Heat both test tubes. In tubes containing vitamin C, the liquid becomes colourless.
The results for the response record.
Reactions to vitamin a (retinol). Vitamin a in the presence of sulfuric acid with chloroform gives a red coloration. In a dry test tube pour a few drops of fish oil and the same of chloroform. Carefully add 3-5 drops of concentrated sulfuric acid. The liquid becomes red. The results for the response record.
Reaction to vitamin D (calciferol).
A) Vitamin D in the presence of hydrochloric acid gives with aniline red staining. In a dry test tube, pour about 1 ml of fish oil and the same mixture of aniline with hydrochloric acid. Stir the contents of the tubes. Carefully heat. The liquid gradually acquires a red color.
B) Vitamin D a solution of bromine in chloroform gives a greenish-blue coloration. In dry test tube put approximately 1 ml of fish oil and the same amount of bromine in chloroform. The liquid is a greenish-blue color.
The results for the response record.
Reaction to the vitamin E (tocopherol). The oxidation of vitamin E nitric acid forms a substance, colored red. In a dry test-tube pour 1 ml of tocopherol solution, add 5 drops of concentrated nitric acid, and shake gently heat the contents of the tube. Red emulsion is formed. The results for the response record.
Laboratory work № 9
Theme: The allocation of ribonucleoproteins from yeast and detection of hydrolysis products of quality responses.
Aim: to study the hydrolysis of RNA and qualitative reactions to the hydrolysis products.
Objectives: to perform experiments, explain the results and draw conclusions.
The selection of the nucleoproteins from a biological material can be accomplished by various methods: by extraction with water or a mild to medium alkaline solutions and salts, followed by precipitation with acetic acid nucleoproteins, ultracentrifugation, gelfiltration. When applying any of them you first need to destroy the cell membrane. The material taken for the study, homogenized. All nucleoproteins can be subjected to hydrolytic decomposition, in which there is a consistent gap first essential, and then the glycosidic linkages. First, the nucleoproteins break down into simple proteins and nucleic acids. Proteins can then be subjected to hydrolysis to peptides and amino acids.
THE PRACTICAL PART
The allocation of ribonucleoproteins
Progress
To obtain ribonucleoproteins in a mortar were placed 10 g of yeast. Add 4 ml water-ether mixture (1:1) and stirred. Then add 5 g of quartz sand and triturated, priliva small portions of 50 ml of 0.4 % NaOH. The resulting mass is triturated for at least 15 minutes, after which the suspension was filtered through a paper filter or centrifuge. Throws the resulting precipitate, and the filtrate (centrifugate) are collected, placed in a beaker and gradually add a 10% solution of acetic acid (5-6 ml). After each addition check the litmus test of the reaction medium. Acid is added until, until the reaction medium becomes slightly acid. In these circumstances, the nucleoproteins precipitates. The resulting suspension is centrifuged, the filtrate is decanted and the precipitate is separated nucleoproteins.
The hydrolysis of nucleoproteins yeast.
To study the chemical composition of nucleoproteins is carried out acid hydrolysis of yeast, rich in nucleoproteins, and identify the products of hydrolysis of the polypeptides, purine bases, carbohydrates and phosphoric acid specific for each substance reactions.
Progress
In a large wide tube (15 х 1,5 cm) was placed 0.5 g of fresh baking or 0.1 g of dry yeast and pour 4 ml of 10% sulfuric acid solution. The tube is closed by a plug, a fridge in which is a glass tube with a length of 25-30 cm, and put on an asbestos net.
1 hour after the start of boiling of the liquid hydrolysis is stopped, allow the contents to cool, filtered and in the filtrate determine the products of hydrolysis of nucleoproteins.
Qualitative reactions for the hydrolysis products of nucleoproteins
Determination of proteins and peptides
When conducting hydrolysis under the conditions set out in clause 4.2, proteins are subject to him only partially and only broken down to peptides. Proteins and peptides in the hydrolysate detected using biuret reaction.
Progress
The biuret reaction to the polypeptides. To 5 drops of hydrolysate, add 10 drops of 10 % solution NaOH and one drop of a 1% solution of copper sulfate. The liquid turns purple.
The opening of purine bases. Silver test for purine bases. 10 drops of hydrolysate is neutralized with one drop of ammonia and add 5 drops of 1% solution of silver nitrate. After 3-5 minutes falls loose brown precipitate of the silver compounds of purine bases (adenine, guanine).
The opening of the pentoses. Detection of pentoses is based on their dehydration concentrated acids to form furfural, which gives with thymol (reaction MOLISA), α-naphthol and orcine colored compounds.
Attention ! These reactions are carried out only with dry test tubes. Ribose and deoxyribose can be opened by reaction with diphenylamine, which with ribose gives a green coloration, and with the deoxyribose gives a blue coloration.
The Reaction Of MOLISA
Progress
Qualitative reaction of MOLISA on ribose and deoxyribose. To the 10 drops of hydrolysate yeast add 2-3 drops of 1 % alcoholic solution of thymol or α-naphthol, mix and on the side of the tube gently pour 20 drops of concentrated sulfuric acid. By shaking the test tube appears red staining due to the formation of the condensation products of furfural with thymol or α-naphthol.
In test tube pour 10 drops of hydrolysate, add 1-2 drops of a 0.1 % solution of α-naphthol and mix well. Then carefully, on the side of the tube, pour 10-15 drops of concentrated sulfuric acid. When standing on the boundary between two phases there is a purple staining.
In test tube pour 10 drops of hydrolysate, add 10-15 drops of orcine and 10-15 drops of concentrated sulfuric acid. The mixture was heated to boiling and observe for staining (green or pink-red).
Opening of the ribose and desoxyribose
Progress
Test Trommer on ribose and desoxyribose. To 5 drops of hydrolysate, add 10 drops of 30 % solution of sodium hydroxide and 1-3 drops of 7 % solution of copper sulfate until a persistent precipitate copper hydroxide. The liquid was stirred and heated to boiling. The result falls a red precipitate of cuprous oxide, or yellow precipitate of hydrate of copper oxide due to oxidation of the ribose and the restoration of the hydroxide of copper in the hydrate of copper oxide.
The detection of phosphoric acid
Progress
The molybdenum test. To 20 drops of molybdenic reagent (solution of ammonium molybdate in nitric acid) add 2-3 drops of hydrolysate and boil for a few minutes on the fire. In the presence of phosphoric acid, the liquid is painted in lemon yellow color. Upon cooling a yellow crystalline precipitate falls complex compounds of phosphorus-ammonium molybdate (NH4)3PO4 x 12MoO3.
The results are entered in table 1:
The test results table 1.
|
The name of the protein complex |
Prosthetic group |
Name reactions that detect components of proteid |
Used reagents, the reaction conditions |
What caused the reaction |
|
|
|
|
|
|
In test tube pour 10 drops of hydrolysate and gradually add concentrated ammonia solution until the sharp smell, and then 10 drops of magnesia mixture. Formed a crystalline precipitate of magnesium phosphate-ammonium MgNH4PO4.
REPORTING
The report shall be prepared stating the purpose, tasks, includes the equations of reactions, experimental data and conclusions.
Laboratory work 10
Theme: Allocation of dezoxiribonukleoproteid from a spleen.
Aim: to obtain DNA from spleen.
Objectives: to perform experiments, explain the results and draw conclusions.
With nucleoproteids the liver, a spleen, a pancreas, kidneys, yeast are rich. They are dissolved in the diluted solutions of alkalis and drop out in a deposit at solution acidulation. Dezoxiribonukleoproteida are well dissolved also in salt solutions.
Reactants and materials: a) a liver or a spleen of cattle or a pig, fresh or frozen; b) chloride sodium, 5 percentage solution; c) a wooden stick with notches.
Experience performance order: 2-2,5 g of a liver or a spleen cut on small slices and then pound in a mortar with the 5th percentage solution of chloride sodium, having added a little glass powder. Solution of salt is added in the small portions (on 10-15 ml), all about 80 ml spend it.
Pound 12-15 minutes before obtaining homogeneous weight. Contents of a mortar are spilled in centrifugal test tubes and centrifuged 10-15 minutes (at 2500 revolutions per minute) then measure the volume of a tsentrifugat (merging it in the glass cylinder),
In a glass pour the distilled water (which volume has to be six times more than the volume of a centrifugat) and, slowly stirring slowly in a glass with a wooden stick, pour in water centrifugat. Dezoxiribonukleoproteida drop out in the form of threads which reel up on a stick.
High-quality reaction to DNA
Existence of DNA is determined by color reactions, characteristic for a dezoksiriboza. Often apply reaction with biphenyl amine (C6H5-NH-C6H5). Biphenyl amine with dezoxiribozy or DNA forms connection of blue color. The ribose and RNA give green coloring with biphenyl amine.
Reactants and materials: a) a deposit of a dezoxiribonukleoproteid (you watch the previous work); b) difenilaminovy reactant: 1 g of biphenyl amine dissolve in 100 ml of ice acetic acid. Add 2,75 g to solution the concentrated chamois of acid (density 1,836); c) the caustic rubbed, 0,4 percentage solution.
Experience performance order: The part of a deposit of a dezoxiribonukleoproteid is transferred to a test tube and add 0,5-1 ml of solution of a caustic natr (before dissolution). To solution flow the equal volume of a difenilaminovy reactant. The deposit which is dropping out in the beginning will be dissolved next portions of a reactant then it is narevat within 15-20 minutes in the boiling water bath. There is a blue coloring.
Laboratory work № 11
Theme: High-quality reactions to difficult proteins
Aim: studying of high-quality reactions to difficult proteins.
Objectives: to perform experiments, explain the results and draw conclusions.
Relevance of a subject: At soft hydrolysis there is rather superficial disintegration of protein. Pirimidin nucleotides at such hydrolysis don't break up, and purin break up with education the purin of the bases (adenine and a guanine), a ribose and phosphoric acid. Carrying out hydrolysis under certain conditions, it is possible to allocate consistently various intermediate products of hydrolytic disintegration of nucleoproteids and to define them by high-quality reactions.
High-quality reactions to proteins and peptides
When carrying out hydrolysis under the conditions specified in laboratory work 9, proteins are exposed to it only partially and are split only to peptides. Proteins and peptides in a hydrolyzate find by means of biuret reaction.
Progress
The biuret reaction to the polypeptides. To 5 drops of hydrolysate, add 10 drops of 10 % solution NaOH and one drop of a 1% solution of copper sulfate. The liquid turns purple.
The opening of purine bases. Silver test for purine bases. 10 drops of hydrolysate is neutralized with one drop of ammonia and add 5 drops of 1 % solution of silver nitrate. After 3-5 minutes falls loose brown precipitate of the silver compounds of purine bases (adenine, guanine).
The opening of the pentoses. Detection of pentoses is based on their dehydration concentrated acids to form furfural, which gives with thymol (reaction MOLISA), α-naphthol and orcine colored compounds.
Attention ! These reactions are carried out only with dry test tubes. Ribose and deoxyribose can be opened by reaction with diphenylamine, which with ribose gives a green coloration, and with the deoxyribose gives a blue coloration.
The Reaction Of MOLISA
Progress
Qualitative reaction of MOLISA on ribose and dezoxyribose. To the 10 drops of hydrolysate yeast add 2-3 drops of 1 % alcoholic solution of thymol or α-naphthol, mix and on the side of the tube gently pour 20 drops of concentrated sulfuric acid. By shaking the test tube appears red staining due to the formation of the condensation products of furfural with thymol or α-naphthol.
In test tube pour 10 drops of hydrolysate, add 1-2 drops of a 0.1 % solution of α-naphthol and mix well. Then carefully, on the side of the tube, pour 10-15 drops of concentrated sulfuric acid. When standing on the boundary between two phases there is a purple staining.
In test tube pour 10 drops of hydrolysate, add 10-15 drops of orcine and 10-15 drops of concentrated sulfuric acid. The mixture was heated to boiling and observe for staining (green or pink-red).
Opening of the ribose and deoxyribose
Progress
Test Trommer on ribose and deoxyribose. To 5 drops of hydrolysate, add 10 drops of 30 % solution of sodium hydroxide and 1-3 drops of 7 % solution of copper sulfate until a persistent precipitate copper hydroxide. The liquid was stirred and heated to boiling. The result falls a red precipitate of cuprous oxide, or yellow precipitate of hydrate of copper oxide due to oxidation of the ribose and the restoration of the hydroxide of copper in the hydrate of copper oxide.
The detection of phosphoric acid
Progress
The molybdenum test. To 20 drops of molybdenic reagent (solution of ammonium molybdate in nitric acid) add 2-3 drops of hydrolysate and boil for a few minutes on the fire. In the presence of phosphoric acid, the liquid is painted in lemon yellow color. Upon cooling a yellow crystalline precipitate falls complex compounds of phosphorus-ammonium molybdate (NH4)3PO4 ∙ 12MoO3.
The results are entered in table 2:
The test results table 2.
|
The name of the protein complex |
Prosthetic group |
Name reactions that detect components of proteid |
Used reagents, the reaction conditions |
What caused the reaction |
|
|
|
|
|
|
|
|
|
|
|
|
In test tube pour 10 drops of hydrolysate and gradually add concentrated ammonia solution until the sharp smell, and then 10 drops of magnesia mixture. Formed a crystalline precipitate of magnesium phosphate-ammonium MgNH4PO4.
Laboratoty work № 12
Тhеme: Qualitative reactions for carbohydrates
Aim: to study the qualitative reactions for carbohydrates.
Objectives: to perform experiments, explain the results and draw conclusions.
1. Qualitative reactions for glucose.
A) Reaction of silver mirror. In test tube pour 2 ml of glucose solution, add half as ammonia solution of silver nitrate and boil out the lamp. On the side of the tube, the mirror is formed as a result of the separation of metallic silver.
The test results and the equation of the reaction is recorded.
B) Reaction of Felling. In test tube pour 2 ml of glucose solution and add an equal amount of Felling's reagent consisting of equal parts of solutions 1 (CuSO4) and 2 (NaOH) containing tartrate sodium-potassium, contributing to the dissolution of the precipitate of hydrate of copper oxide. Boil out the lamp. Appears brick-red staining resulting from copper oxide.
The test results and the equation of the reaction is recorded.
B) Reaction оf Nylander. In test tube pour the same amount of glucose solution, as in the previous experiments, and add 5-6 drops of Nylander's reagent (consists of basic bismuth nitrate, caustic soda, and the salt sеgnet; in the interaction of the first two reagents is formed a hydrate of bismuth oxide, held in solution the salt sеgnet). Boil for 2-3 minutes. The contents of the tube turns black due to the separation of metallic bismuth.
The test results and the equation of the reaction is recorded.
2. The Selivanov's reaction to fructose.
In test tube pour 2 ml of a solution of fructose. Add 5 drops of Selivanov's reagent (0.5 g resorcinol in 100 ml of 20 % HCl solution) and boil out the lamp. Becomes cherry-red colouring.
The test results and the equation of the reaction is recorded.
3. The reaction of Tollens on the pentose.
In test tube pour 2 ml of a solution of any pentose, add an equal volume of 20 % HCl solution and 5 drops of 0.5 % solution floroglûcina. Boil for 1 minute. As a result of interaction of the formed furfural with phloroglucinol appears red staining.
In the absence of pentoses can be used to experience wood sawdust containing pentosans (polysaccharides, built of pentoses). In this case, use a solution of concentrated hydrochloric acid. When this occurs the hydrolysis of the pentosans. Then add a few crystals of floroglûcina, and after a few minutes, there is a red staining.
4. Reducing properties of disaccharides.
3 the test tube is poured 2 ml of a solution of disaccharides: the first is sucrose, the second – maltose, in the third – lactose. With all three produce the reaction of Felling (as described above).
The test results and the equation of the reaction is recorded.
5. The opening of fructose in the composition of the disaccharides.
As in the previous experience, it is poured into a test tube solutions of sucrose, maltose and lactose. With all three solutions produce Selivanov's reaction.
The test results and the equation of the reaction is recorded.
6. Acid hydrolysis of sucrose.
In test tube pour 4 ml of the sucrose solution, add 1 ml of a 1 normal solution of sulphuric acid and boil on a spirit lamp for 2 minutes. The solution was neutralized with 1 ml of 2 normal sodium hydroxide solution, add an equal volume of Felling's reagent and boil the lamp.
The test results and the equation of the reaction is recorded.
7. Qualitative reactions for polysaccharides.
In one test tube put 1 ml of starch solution, and in the other the same amount of a solution of glycogen in each and add 1-2 drops of Lugol's solution (iodine solution in potassium iodide). See the coloration.
The test results and the equation of the reaction is recorded.
8. The precipitation of the glycogen with alcohol.
Glycogen in water forms a colloidal solution, which is hydrophilic Sol. Therefore, a substance that takes water means violate its stability.
In test tube pour 1 ml of glycogen solution and add 10 ml of alcohol. The solution immediately became cloudy, and after some time a solid precipitate.
The test results and the equation of the reaction is recorded.
Laboratory work № 13
Theme: Separation of lipids from muscle tissue and quality detection
Aim: to obtain lipids from the biological material and to study qualitative reaction.
Objectives: to perform experiments, explain the results and draw conclusions.
Methods of extraction of fat.
Typically, the lipids extracted from dried (dehydrated) tissues relevant organic solvents (alcohols, ethers, benzene, toluene, gasoline, acetone, pyridine, chloroform, carbon tetrachloride, carbon disulfide, petroleum ether, etc.). For the separation of lipids using their different solubility in various solvents: some of these are soluble in ether but poorly in acetone (e.g., phospholipids), the other soluble in benzene, but insoluble in alcohol (cholesterol, cerebroside), etc. back fat (oil) is extracted easily. To retrieve the related lipids only after the destruction of protein-lipid complexes. Lipids from the bound state to a free state is transferred either through the use gidrolizuemye funds, or by prior boiling of the material with alcohol. In the latter case, the lipids are excreted in unchanged form.
The most simple method of determination of total lipids in the tissues is the method of long insisting hinge-plate fabrics in chloroform - methanol mixture. On a difference of weights of sample before and after extraction found percentage lipids.
In the allocation of lipids from biological materials is their oxidation and degradation, leading to the formation of by-products. Therefore, the selection of lipids should be carried out quickly, in conditions excluding the influence of factors such as increased temperature, oxygen, light, contamination by trace metals, etc.
For the extraction using mixture of methanol and chloroform, which destroys lipoprotein complexes and thus gives an opportunity to fully extract the lipids.
Qualitative reaction for fats and oils.
The drop of oil on the hourly or slide add one drop of a 1% solution of osmium acid. The oil turns black. In addition to the osmium acid, as a reagent on the oil used the dye Sudan III, which stains oil in different shades of red.
For the qualitative detection of oil products in the tissue sections (plant or animal), moistened with one of these reagents and observed under the microscope: a drop of oil in the tissues are colored in the corresponding color (black or osmium acid in red - from the Sudan III).
Detection of glycerol in fats (aсroleina sample)
In test tube add 2-3 drops of oil (fat) and add five times the amount of anhydrous potassium hydrogen sulfate. Heat the test tube gently but strongly (in fume cupboard) until dense white fumes. Note (carefully!) the sharp irritating odor of acrolein. If these couples add a piece of filter paper dampened with ammonia solution of silver oxide, the paper will turn black. Filter paper moistened with a solution faxencerie acid, approached to the neck of the tube, is gradually painted in a pinkish-purple color.
You can repeat this experience with wax instead of oil. The acrolein in this case are not formed.
Akroleina test performed to detect lipid solubility of glycerol. By heating glycerol in the presence of substances that take away water means (a hydrous sulfate of potash, magnesium sulfate, boric acid) the formation of an acrylic unsaturated aldehyde - acrolein:

The definition of saturation (unsaturation) of fats.
Unsaturation of the fat depends on the presence in its composition of unsaturated fatty acids. Unsaturated compounds, as is well known, easy to enter into the reactions of addition, oxidation and polymerization. Usually the degree of unsaturation determine the iodine number. Iodine number is measured by the number of grams of iodine that is attached to 100 grams of fat.
To determine the iodine number of the titrated solution of the fat in chloroform (0,5 grams of fat in 3 ml of chloroform) of 0,001 n solution of iodine in chloroform until a distinct pink color.
Laboratory work № 14
Тhеme: Determination of properties of lipids.
Aim: to study the properties of lipids.
Objectives: to perform experiments, explain the results and draw conclusions.
Qualitative tests for fats. The formation of oil stains. A drop of oil applied with a glass rod on a piece of paper. Spot is formed, does not disappear when heated.
The solubility of fats.
Reagents: a) vegetable oil (sunflower, flax, cotton or other); b) tallow (mutton, beef); c) dietil ether; d) acetone, d) ethanol; e) distilled water.
Put two rows of 4 tubes each. In the tubes of the first row: put a few drops of vegetable oil; in the tubes of the second row – piece of solid fat. In the first test tube of each row pour 2 ml of distilled water, the second poured the same amount of diethyl ether, then poured acetone in a fourth pour of alcohol. All tubes are shaken and see the fat solubility in various solvents. Swab with alcohol, it is recommended to heat in a water bath. Record the results of experience.
Emulsification of fatty oils.
Reagents: a) vegetable oil; b) sodium carbonate, 2 % solution; b) soap, 2 % solution; d) bile; d) distilled water.
In four test-tubes put 5 drops of oil. In the first test tube add 2 ml of distilled water in another test tube add 2 ml of 2% restora sodium carbonate (soda), in the third test tube add the same 2 % solution of soap, in the fourth test tube add 2 ml of water and a few drops of bile. All tubes are shaken and observe the formation in the first test tube unstable emulsion of oil in water, rapidly exfoliating when standing, while others see the formation of stable emulsions due to the action of added emulsifiers, which are adsorbed in the outer layer of fat caped and lower their surface tension.
Aсrolein reaction.
Using sample to determine the presence of acrolein of glycerol in fats. When heating fat with potassium acid sulfate (KHSO4), sodium acid sulfate (NaHSO4) or boric acid (H3BO3) cleavage occurs from a molecule of glycerol with two molecules of water and the formation of acrylic aldehyde, or acrolein, having a pungent irritating odor (burnt fat):
Reagents: a) vegetable oil or animal fat; b) beeswax; C) acid sulfate or potassium acid sulfate sodium crystalline; g) boric acid, crystalline.

In dry test tube add a few drops of vegetable oil or a piece of animal fat, add a little acid powder potassium sulfate or boric acid and gently heated. See white vapours of acrolein, having a pungent odor. Repeat the reaction with the wax – acrolein is not formed, since glycerin is not included in the composition of the waxes.
Laboratory work № 15
Theme: The secretion of cholesterol from the brain. Qualitative detection of cholesterol.
Aim: To be able to determine the cholesterol concentration in blood serum and to interpret this index, to determine the concentration of ketone bodies in urine and interpret the result.
Professional orientation of students:
The increase of ketone bodies contents in blood and in urine is the index of fat catabolismin activation in an organism and insufficient use of ketone bodies as alternative energetic substrate in tissues. This is the reason of development of ketosis at starvation, diabetes mellitus, thyrotoxicosis etc.
The contents of phospholipids in blood is the important index of functional state of liver, possibilities of transport and metabolism of cholesterol. The increase of cholesterol in blood occurs in the prevailing of animal fats and carbohydrates in a diet, as well as in smoking, alcoholism etc.
Methodology of Practical Class.
I. Cholesterol.
Work 1. Qualitative detection and quantitative determination of ketone bodies in urine
1. The qualitative detection of acetone (Legal reaction).
Acetone and acetoacetic acid with sodium nitroprussicum in alkaline medium form red colored compounds. In adding of the concentrated acetic acid the cherry-red complex salt is formed.
Materials and reagents. Urine containing ketone bodies, normal urine, 10 % solution of sodium nitroprussicum, concentrated acetic acid, 10 % solution of NaOH.
Protocol.
Add into two tubes on 0,5 ml of urine: into the 1-st tube – urine of healthy person; into the 2-nd – urine containing ketone bodies. Into both tubes add 0,5 ml of 10 % solution NaOH and 5-7 drops of 10 % solution of sodium nitroprussicum. Observe the appearance of red color in the 2-nd tube. Add several drops of concentrated acetic acid. Red color is changed to cherry color.
2. The qualitative detection of acetone (Herhardt reaction).
In adding of FeCl3 solution to urine containing ketone bodies the sediment is formed.
Materials and reagents. Urine consisting ketone bodies, normal urine, 10 % solution FeCl3.
Protocol.
Add into two tubes 2 ml of urine: into the 1-st tube – urine of healthy person; into the 2-nd – urine containing ketone bodies. Into both tubes add several drops of 10 % solution FeCl3. Observe the formation of sediment.
The quantitative determination of ketone bodies in urine.
If urine contains acetone, the violet ring is formed in the border between layer of ammonia and urine containing sodium nitroprussicum and concentrated acetic acid. The velocity of ring appearance depends on the concentration of acetone in urine. The appearance of ring between 3 and 4 min means that concentration of acetone is 0,0085 g/l.
Materials and reagents. Urine containing ketone bodies, 50 % solution of ammonia sulfate, 10 % solution of sodium nitroprussicum, concentrated acetic acid.
Protocol.
Take 8 tubes. Add into each tube (except the first one) 1 ml of water. Into the 1st and 2nd tubes add 1 ml of urine. Thus, in the 1-st tube the urine is not diluted and in the 2-nd one – two fold diluted. Replace 1 ml of mixture from the 2-nd tube into the 3-rd one, from the 3-rd into the 4-th and so on. Remove 1 ml of mixture from the last tube. Thus, the dilution in the series of tubes will be in 2, 4, 8 and etc. times. Add into each tube 8 drops of ammonia sulfate, 8 drops of 10 % solution of sodium nitroprussicum and 8 drops of concentrated acetic acid. Mix the content of each tube. Carefully (don’t mix) along the walls of tubes pour 1 ml of concentrated ammonia. Note the tube where the violet ring between layers of liquids appears between 3 and 4 min.
Calculation.
Use the formula:
X = 8,5 x a x 1,5,
where
X – concentration of acetone in urine (mg/day);
a – dilution of urine.
Work 1. Qualitative detection of cholesterol.
Materials and reagents. The solution of cholesterol in chloroform; concentrated sulfuric acid; acetic anhydride.
Protocol.
Add into the dry tube 1 ml of chloroform solution of cholesterol, 5-6 drops of acetic anhydride and 1-2 drops of concentrated sulfuric acid. Mix the sample carefully. Observe the appearance of green color.
Work 2. The quantitative determination of cholesterol in blood serum.
Cholesterol in the presence of acetic anhydride and mixture of acetic and sulfuric acid form the green colored compound. The cholesterol concentration is proportional to the intensity of color.
Materials and reagents. Blood serum, reagent N1 (mixture of acetic acid, acetic anhydride and sulfuric acid – 1:5:1), standard solution of cholesterol.
Protocol.
Add into two dry tubes 2 ml of reagent N1. Add into 1-st tube 0,1 ml of standard solution of cholesterol and into 2-nd – 0,1 ml of blood serum. Stir up the tubes and stay in darkness for 20 min. The green color appears. Colorimeter the samples using red filter and water as control.
Calculation:
X = Eexp / Est x Cst (mmol/l).
Eexp – extinction of sample containing blood plasma;
Est - extinction of sample containing standard solution;
Cst – cholesterol concentration in standard solution.
25

шағым қалдыра аласыз